Understanding the Role of All-Trans Retinoic Acid in Acute Myeloid Leukemia Cells
DOI:
https://doi.org/10.14740/wjon2676Keywords:
AML, Differentiation, ATRAAbstract
Background: All-trans retinoic acid (ATRA) has revolutionized the management of acute promyelocytic leukemia (APL) and has generated interest in differentiation therapy as means to reduce reliance on conventional chemotherapy in other subtypes of acute myeloid leukemia (AML). However, the precise relationship between immunophenotypic changes and morphological maturation during ATRA-induced differentiation remains poorly defined. This study aimed to investigate the phenotypic and morphological progression of HL60 cells treated with ATRA and to establish quantitative correlations between surface marker expression and cell morphology.
Methods: HL60 cells were treated with 1 µM ATRA. Expression of myeloid and monocytic surface markers (CD38, CD45, CD11b, human leukocyte antigen (HLA)-DR, CD15, CD13, CD33) was analyzed in parallel with morphological assessment using Giemsa-stained cytospin preparations. Pearson correlation analysis was applied to link surface marker expression with specific stages of granulocytic differentiation. In addition, the mRNA expression of myeloid-specific transcription factors PU.1, RUNX1 and CEBPα was evaluated following ATRA treatment.
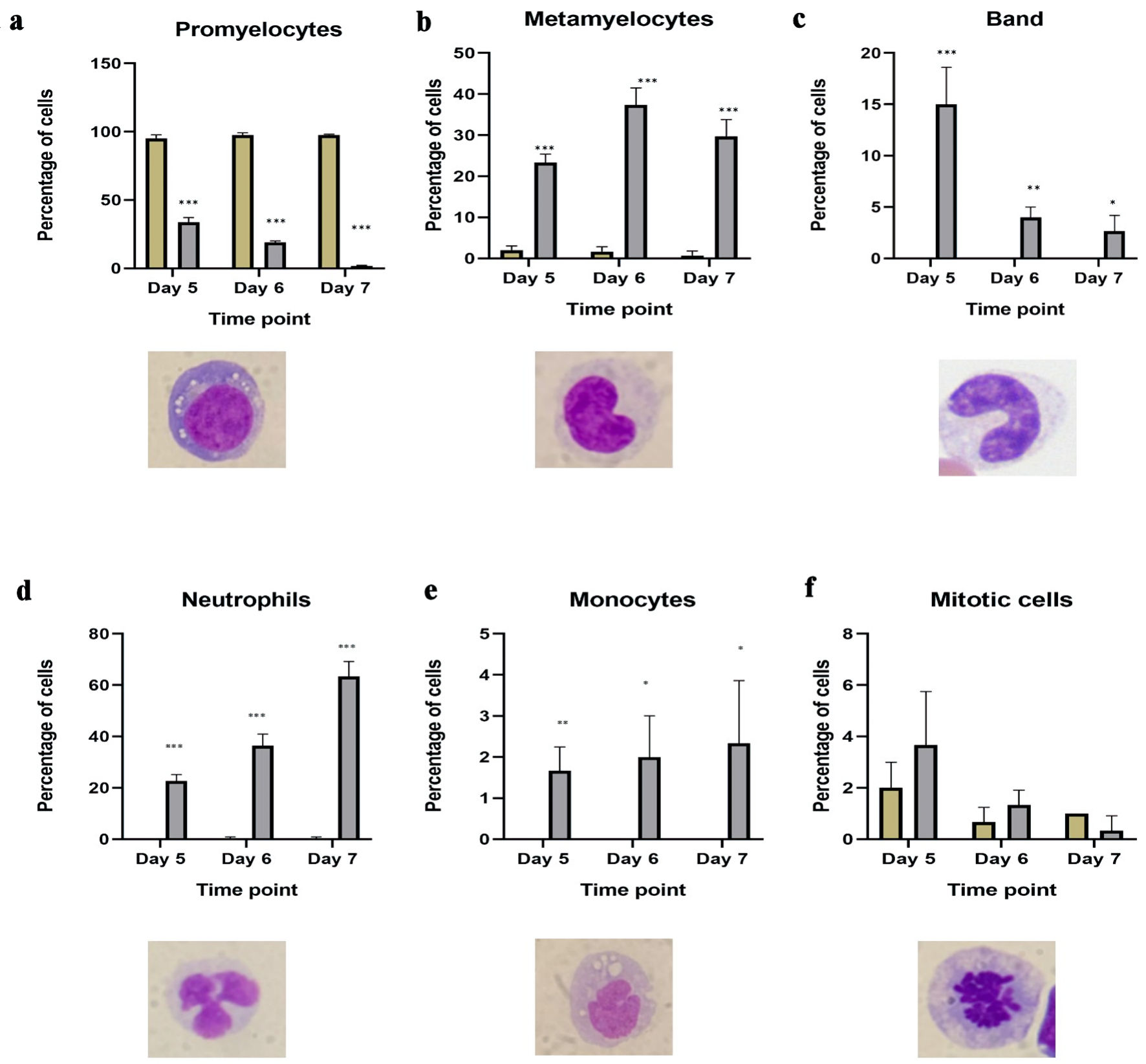
Results: ATRA induced a stepwise maturation of HL60 cells from promyelocytes through metamyelocytes and band forms to segmented neutrophils, recapitulating physiological granulopoiesis. Acquisition of mature morphology correlated positively with CD38, CD45, CD11b, HLA-DR, and CD15 expression, while CD13 and CD33 were progressively downregulated and inversely correlated with differentiation. Importantly, this study established quantitative links between distinct immunophenotypic signatures and defined morphological stages, a relationship not clearly demonstrated in previous HL60 studies. Concomitantly, ATRA upregulated transcription factors PU.1, RUNX1 and CEBPα, supporting the coordinated regulation of phenotypic and morphological maturation.
Conclusions: Our study reveals that ATRA not only promotes broad differentiation of HL60 cells but also orchestrates a targeted reprogramming that synchronizes morphological maturation with distinct immunophenotypic shifts, mediated by transcriptional upregulation of PU.1, RUNX1 and CEBPα. This integrative profiling - linking molecular, functional, and morphological parameters - offers a nuanced understanding of the differentiation trajectory and establishes a robust framework for assessing ATRA and other differentiation-based strategies in AML. By delineating these correlations, our findings pave the way for therapeutic approaches that leverage controlled leukemic cell maturation, potentially minimizing the cytotoxic burden associated with conventional chemotherapy.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









