Clinical Utility of Targeted Next-Generation Sequencing for Determining Human Epidermal Growth Factor Receptor 2 Status and Optimizing Targeted Therapy in Breast Cancer
DOI:
https://doi.org/10.14740/wjon2583Keywords:
Breast cancer, Copy number alteration, Human epidermal growth factor receptor 2, Next-generation sequencing, Precision medicineAbstract
Background: The development of targeted next-generation sequencing (NGS) technologies has contributed to precision medicine, as evidenced by the growing interest in evaluating human epidermal growth factor receptor 2 (HER2) expression status to treat unresectable/metastatic HER2-low breast cancer (BC). However, the concordance between erb-b2 receptor tyrosine kinase 2 (ERBB2) copy number alteration (CNA) and HER2 immunohistochemistry (IHC) has never been determined. The aim of this study was to evaluate the utility of targeted NGS for determining HER2 status and optimizing targeted therapies for BC.
Methods: ERBB2 CNAs were examined by targeted NGS in 41 formalin-fixed paraffin-embedded (FFPE) BC tissues. ERBB2 CNA was compared with HER2 status evaluated by IHC in tissue sections, which were identical to those subjected to targeted NGS, using the Ventana 4B5 antibody.
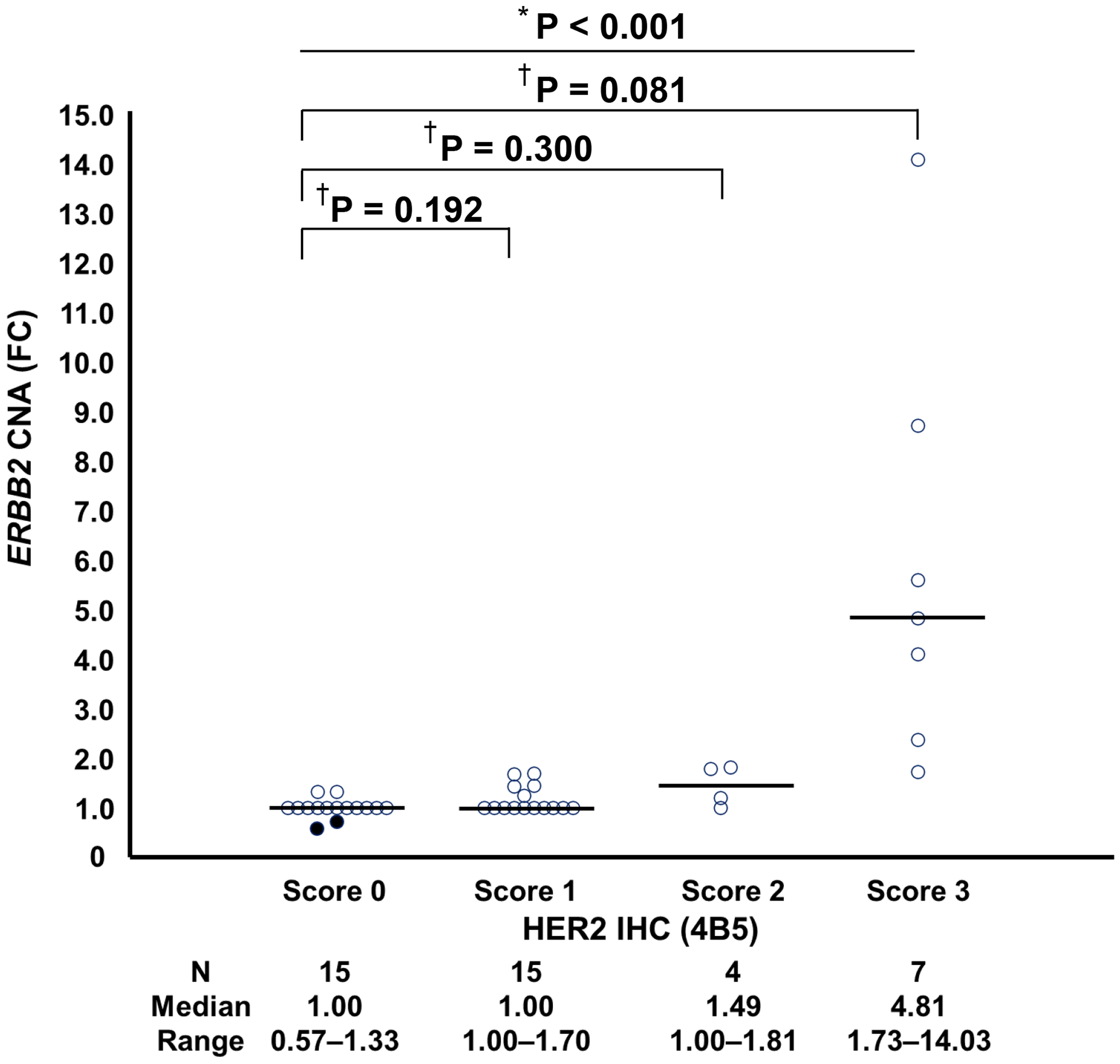
Results: The median fold changes (FCs) for ERBB2 CNAs in tumors with an IHC score of 3+, 2+, 1+, and 0 were 4.81, 1.49, 1.00, and 1.00, respectively. The difference in the FC for ERBB2 CNA according to HER2 status was statistically significant (P < 0.001). An FC greater than 1.0 for ERBB2 CNA was established as the cutoff value to differentiate between tumors with an IHC score of 3+, 2+, or 1+ and tumors with an IHC score of 0, on the basis of receiver operating characteristic curve analysis. The overall percent agreement, positive percent agreement, negative percent agreement, and Cohen’s kappa between ERBB2 CNA and HER2 status were 68.3%, 57.7%, 86.7%, and 0.39, respectively. The numbers of patients with mutations in ERBB2, estrogen receptor 1 (ESR1), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), serine/threonine kinase 1 (AKT1), and phosphatase and tensin homolog (PTEN) were 7, 3, 6, 1, and 5, respectively. Targeted NGS detected additional gene mutations and presented treatment options for seven of 22 patients (31.8%) with an FC of ERBB2 CNA = 1.00.
Conclusions: Targeted NGS has the potential in distinguishing HER2 IHC 3+, 2+, and 1+ tumors from IHC 0 in patients with BC; however, differentiating between HER2 IHC 1+ and 0 remains challenging. Additionally, targeted NGS may aid in the identification of actionable mutations, thereby contributing to the selection of optimal treatment strategies in BC management.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.