Efficacy of Infliximab Versus Vedolizumab in the Management of Immune Checkpoint Inhibitor-Induced Colitis: A Systematic Review and Meta-Analysis
DOI:
https://doi.org/10.14740/wjon2613Keywords:
Vedolizumab, Immune-mediated colitis, Infliximab, Steroids, PDL-1, CTLA-4Abstract
Background: Immune checkpoint inhibitors (ICIs) can cause severe gastrointestinal immune-related adverse events (irAEs), often leading to treatment interruption and increased morbidity. Immune-mediated colitis (IMC) ranges from mild diarrhea to life-threatening colitis, sometimes requiring urgent intervention. While corticosteroids are the first-line treatment, selective immunosuppressive therapy (SIT) with either infliximab or vedolizumab is used for steroid-refractory or dependent cases. However, standardized practices are lacking, and treatment decisions are largely left to provider discretion. This study compares infliximab and vedolizumab for IMC, focusing on remission rates, recurrence, SIT dosing, and systemic steroid exposure duration.
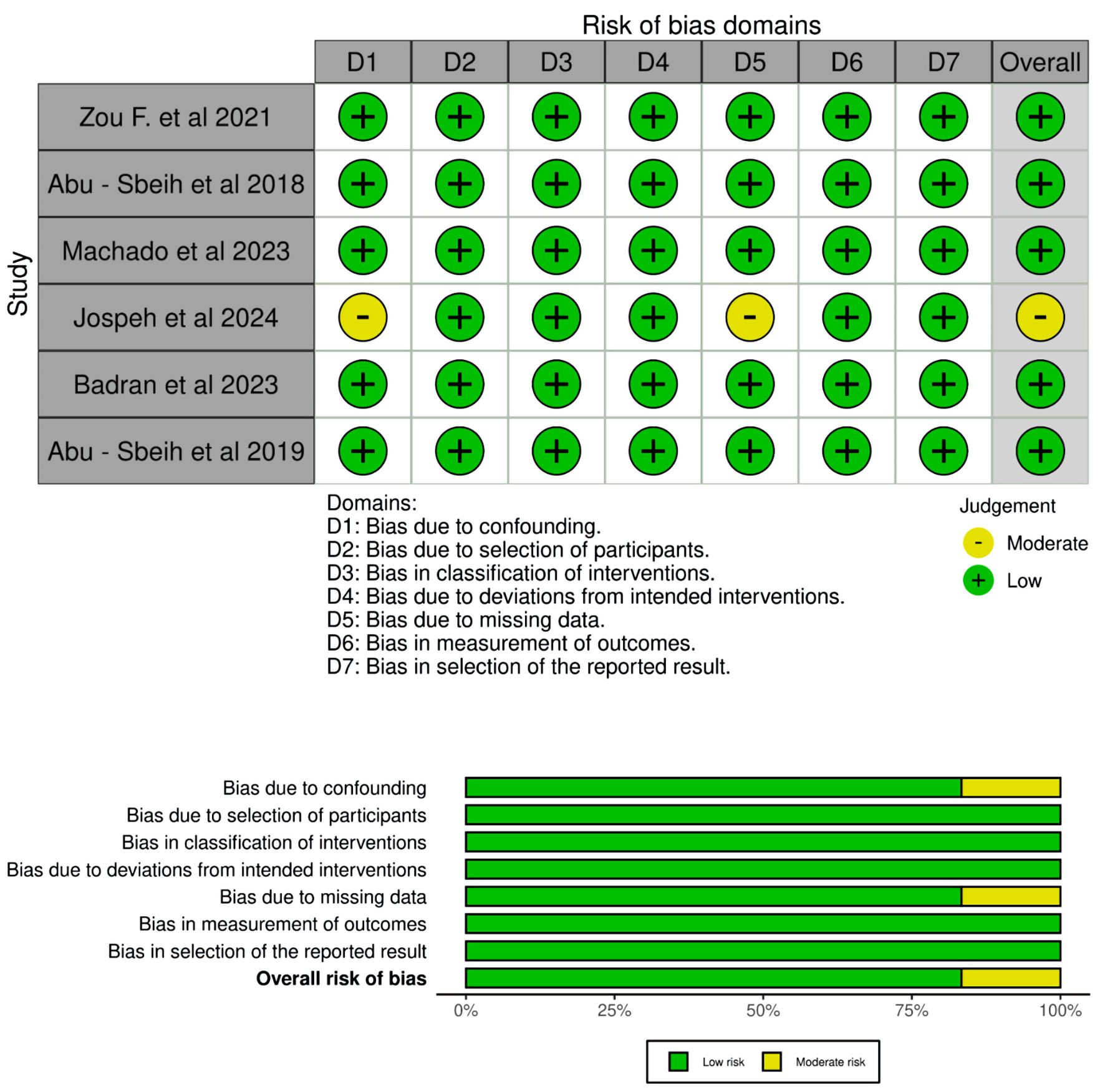
Methods: We identified six retrospective cohort studies that compared infliximab with vedolizumab in the treatment of IMC through a systematic search of PubMed, EMBASE, Cochrane Library, Scopus, CINAHL, Google Scholar, and Web of Science in English from inception until October 2024. From the identified literature, we extracted pertinent data such as remission and recurrence of IMC. Pooled analysis and heterogeneity analysis were performed using R Studio version 4.4.1. The risk of bias was assessed using the Newcastle-Ottawa Scale.
Results: A total of six studies with 645 patients were included. In ICI-associated colitis, vedolizumab was associated with lower recurrence rates (odds ratio (OR): 0.29, 95% confidence interval (CI): 0.15 - 0.54) and shorter systemic steroid exposure (mean difference (MD): -16.88 days, 95% CI: -20.47 to -13.30) compared to infliximab. While vedolizumab showed improved remission, there was no statistically significant difference in remission rates between vedolizumab and infliximab monotherapy (OR: 3.16, 95% CI: 0.29 - 34.01). Remission was achieved with fewer doses of infliximab than vedolizumab (MD: 1.16, 95% CI: 0.09 - 2.22). The mean number of vedolizumab doses was 2.57 (raw mean score (MRAW): 2.57, 95% CI: 1.43 - 2.71), while the mean number of infliximab doses was 1.36 (MRAW: 1.36, 95% CI: 0.69 - 2.02).
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.