Strong Signals of Adverse Events in Tyrosine Kinase Inhibitor Therapy for Liver Cancer Treatment
DOI:
https://doi.org/10.14740/wjon2685Keywords:
Adverse events, Strong signals, Tyrosine kinase inhibitors, Liver cancer, Hepatocellular carcinoma, FDA Adverse Event Reporting SystemAbstract
Background: This study was to identify strong adverse event (AE) signals associated with four tyrosine kinase inhibitors (TKIs) (sorafenib, regorafenib, lenvatinib, and cabozantinib), and compare these signals with regulatory drug facts from multiple global agencies.
Methods: Data from the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS, 2007 - 2024) were analyzed. Each AE was treated as a binary variable, and logistic regression with robust error estimation was used to identify strong signals (odds ratio > 2, lower 95% confidence interval > 1). AE signals were compared with drug facts from the FDA (USA), European Medicines Agency (EMA, European Union), Pharmaceuticals and Medical Devices Agency (PMDA, Japan), and National Medical Products Administration (NMPA, China).
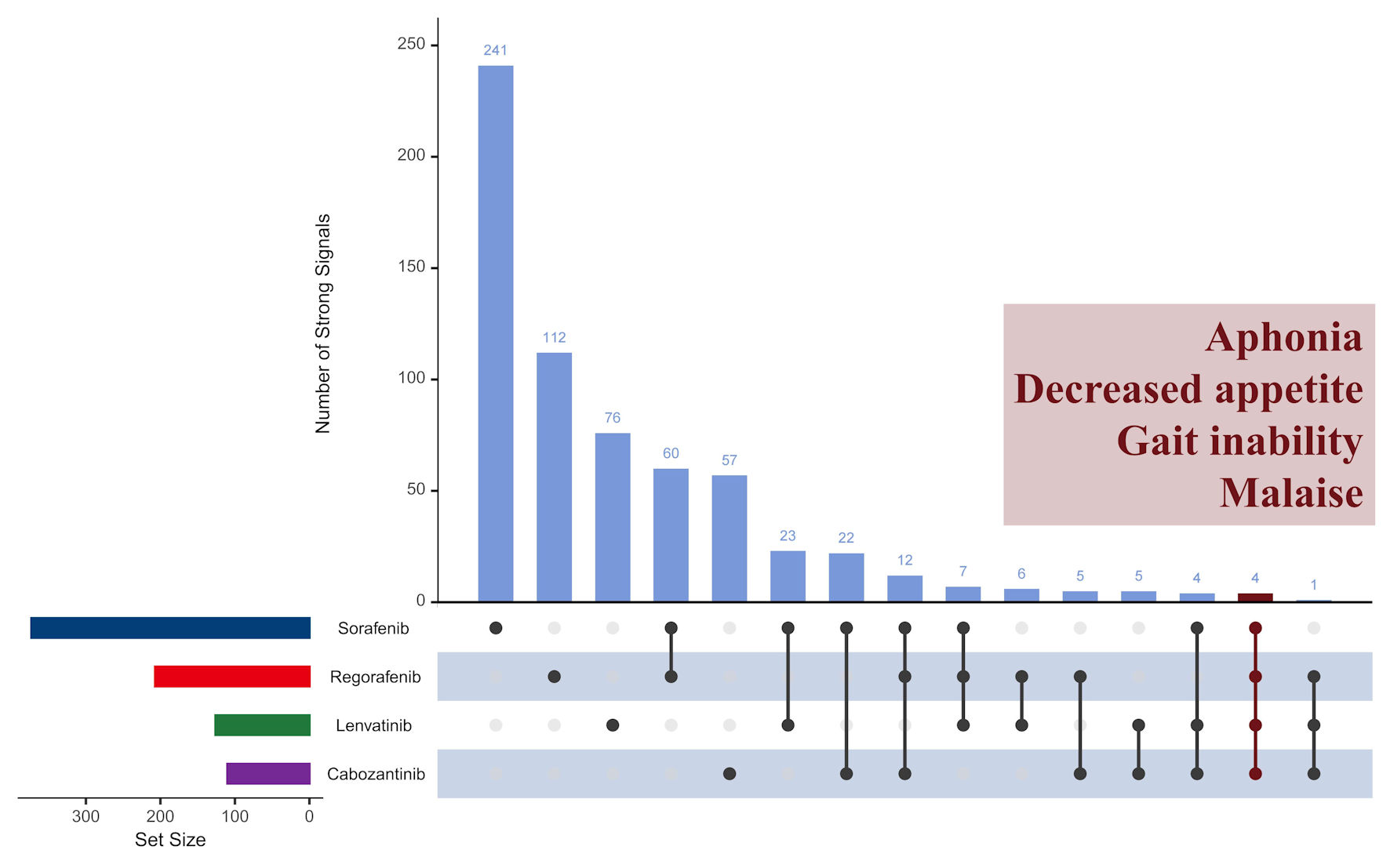
Results: Among 33,801 identifiers (137,345 records), 816 strong AE signals were found. Sorafenib had the most (373), followed by regorafenib (207), lenvatinib (126), and cabozantinib (110). Notable AEs included pharyngeal hemorrhage (sorafenib), retinal artery occlusion (regorafenib), intracranial aneurysm (lenvatinib), and mood swings (cabozantinib). Thirty-two signals had a 100% likelihood of critical outcomes, with no overlap across drugs. AEs were more frequent in males and older populations. Significant discrepancies in AE profiles were observed among regulatory agencies, with low overlap between FAERS and agency data.
Conclusions: This study provides a comprehensive analysis of AE signals for four TKIs in liver cancer, identifying associations rather than causal relationships. The findings highlight significant variation in AE profiles and discrepancies between clinical trial data and real-world evidence. These results are hypothesis-generating and emphasize the need for personalized treatments, enhanced monitoring and intervention, and improved global AE reporting, while acknowledging the inherent limitations of spontaneous reporting systems.
Published
Issue
Section
License
Copyright (c) 2026 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









