Clinical Significance and Potential Molecular Mechanisms of Angiotensin-Converting Enzyme 2 in Colorectal Cancer
DOI:
https://doi.org/10.14740/wjon2650Keywords:
Angiotensin-converting enzyme 2, Colorectal cancer, Immune microenvironment, Immunosuppressive, Nerve invasion, PD-L1, CD8, Mucinous adenocarcinoma, NRAS (Q61R/L/H/K) mutationAbstract
Background: Angiotensin-converting enzyme 2 (ACE2) exhibits tumor-suppressive potential in cancers, but its role in colorectal cancer (CRC) is unclear. The aim of the study was to investigate ACE2 expression, clinical significance, and immune microenvironment associations in CRC.
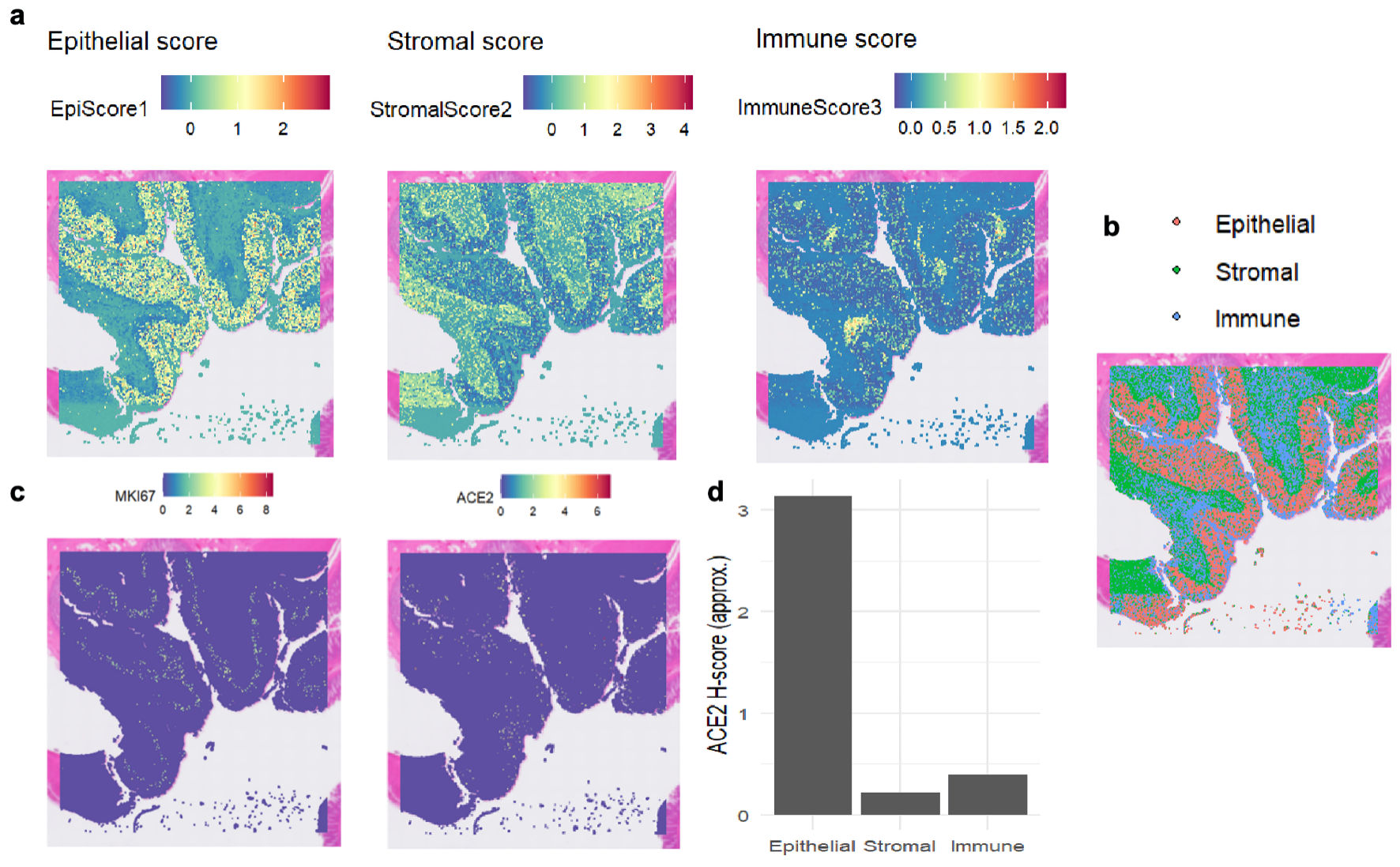
Methods: A multidimensional approach was taken using single-cell RNA sequencing and spatial transcriptomics to analyze ACE2 expression in CRC cells. High-throughput data from the Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA) (2,275 CRC and 1,269 adjacent tissues) were used to assess mRNA levels. Immunohistochemistry was performed to examine ACE2 protein expression in 66 CRC and 75 adjacent tissues. Molecular testing assessed associations with Kirsten rat sarcoma viral oncogene homolog (KRAS), neuroblastoma RAS viral oncogene homolog (NRAS), and B-Raf proto-oncogene, serine/threonine kinase (BRAF) mutations. Immune infiltration was analyzed using single-sample gene set enrichment analysis (ssGSEA), focusing on 24 immune cell types, CD8+ T cells, and programmed death ligand 1 (PD-L1) correlations.
Results: ACE2 was highly expressed in malignant cells and Ki-67-activated regions. mRNA and protein levels were upregulated in CRC (standardized mean difference (SMD) = 0.321, area under the curve (AUC) = 0.844). High ACE2 exhibited significant associations with nerve invasion, lower expression in mucinous adenocarcinomas, and NRAS (Q61R/L/H/K) mutations. ACE2 negatively showed an inverse correlation with CD8+ T-cell infiltration (r = -0.186, P < 0.001) and PD-L1 expression (r = -0.282, P = 0.022).
Conclusions: The upregulation of ACE2 is associated with nerve invasion, pathological type, and an immunosuppressive microenvironment with reduced CD8+ T-cell infiltration and PD-L1 expression.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution 4.0 International License.









