A Tertiary Lymphoid Structure-Related Gene Signature Predicts Prognosis and Treatment Response in Hepatocellular Carcinoma
DOI:
https://doi.org/10.14740/wjon2646Keywords:
Single-cell sequencing, Tertiary lymphoid structures, Hepatocellular carcinoma, Tumor immune microenvironment, Bioinformatics analysisAbstract
Background: Hepatocellular carcinoma (HCC) carries a poor prognosis with limited treatment options. Tertiary lymphoid structures (TLS) impact tumor immunity, but their role in HCC requires clarification. This study aimed to develop and validate a TLS-related gene signature for predicting survival and therapeutic response in HCC, and to explore its mechanisms.
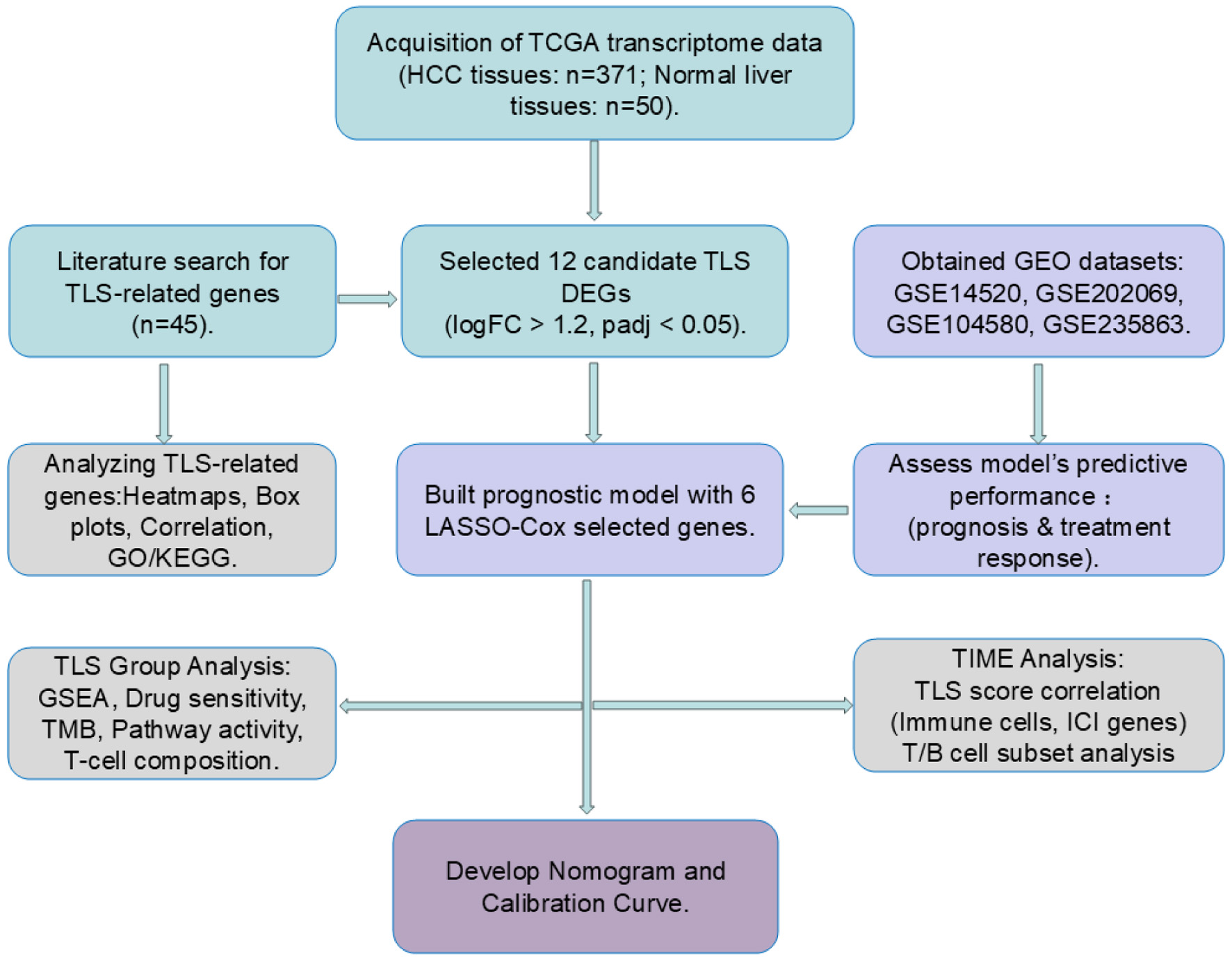
Methods: We analyzed transcriptomic data from public databases (The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO)) using LASSO-Cox regression to identify a six-gene TLS signature (CCL20, CD200, PLAC8, DNASE1L3, C7, and SKAP1). We validated this TLS score across multiple independent HCC cohorts, including patients receiving transarterial chemoembolization (TACE), programmed cell death protein-1 (PD-1)/ligand 1 (PD-L1) inhibitors, or lenvatinib. Through single-cell RNA sequencing (scRNA-seq), we characterized immune microenvironment differences between score groups.
Results: The TLS score effectively stratified patients’ survival outcomes across all validation cohorts. Low TLS scores significantly correlated with improved overall survival, enhanced therapeutic response (especially to immune checkpoint inhibitors (ICIs)), and lower immune evasion potential. Mechanistically, scRNA-seq revealed distinct immune microenvironments: low-score tumors were enriched in cytotoxic and exhausted CD8+ T cells (Tex), favorable for immunotherapy, showing beneficial immune remodeling post-treatment (decreased Tex, increased effector memory T cells). High-score tumors featured dense regulatory T-cell (Treg) infiltration, contributing to immunosuppression.
Conclusion: Our findings suggest a potential TLS-based biomarker for HCC prognosis and therapeutic response. This work offers preliminary insights into tumor immune microenvironment (TIME) heterogeneity, which may be modulated by the Treg/Tex balance, and proposes a possible tool for improving patient stratification.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









