Adjuvant Therapy Benefits for Patients With Human Epidermal Growth Factor Receptor 2-Positive T1aN0M0 Breast Cancer: A Systematic Review and Meta-Analysis
DOI:
https://doi.org/10.14740/wjon2578Keywords:
Breast cancer, HER2-positive, Stage T1aN0, Adjuvant therapy, Survival, Systematic review, Meta-analysisAbstract
Background: While the prognosis for patients with human epidermal growth factor receptor 2 (HER2)-positive pT1a-bN0M0 breast cancer is generally favorable, the optimal approach to personalize adjuvant treatment for T1a tumors remains unclear, which prompted an impetus to conduct a systematic review and meta-analysis for the latter group.
Methods: We examined the literature for studies that provided relevant data about HER2-positive T1a patients. Patient and disease characteristics, therapy details, and survival outcomes were extracted.
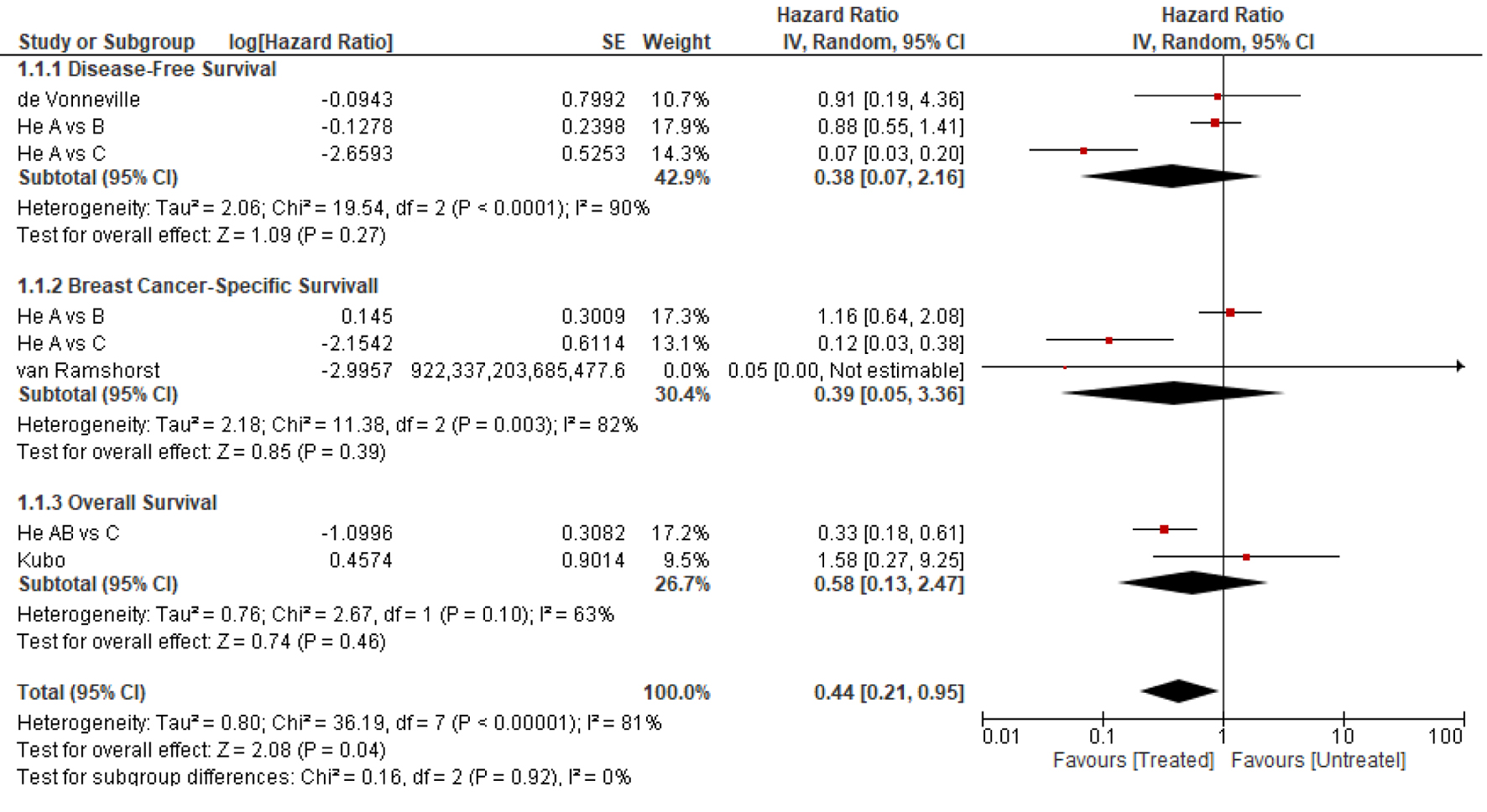
Results: Thirteen studies with 2,089 patients were eligible; four were prospective and nine were retrospective. In the studies where patients did not receive chemotherapy or anti-HER2 therapy, the prognosis was generally favorable, with disease-free survival (DFS) and overall survival of approximately 92% to 99%. Studies comparing treated versus untreated patients showed a survival benefit that varied between 2% and 15%, favoring adjuvant therapy without reaching statistical significance. In the only included randomized trial where all patients received adjuvant paclitaxel and trastuzumab, 10% demonstrated 5-year invasive DFS events. A meta-analysis of four studies showed a nonsignificant survival advantage trend among treated patients. There was inconsistency about the prognostic role of the co-existing hormone receptor status.
Conclusion: Patients with HER2-positive T1aN0 have a favorable prognosis; the benefit of adjuvant chemotherapy plus anti-HER2 varied and showed no convincing statistically significant benefit. The decision to offer adjuvant therapy should balance the expected benefits and risks. Prospective trials that include this population should be able to identify who should receive adjuvant therapy and determine the magnitude of benefit.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.