Prognostic Significance of Post-Neoadjuvant Chemotherapy Carbohydrate Antigen 19-9 Levels in Patients With Resectable Pancreatic Cancer Treated With S-1 and Gemcitabine: A Retrospective Cohort Study
DOI:
https://doi.org/10.14740/wjon2563Keywords:
Pancreatic cancer, CA19-9, Neoadjuvant therapy, NATGSAbstract
Background: Carbohydrate antigen 19-9 (CA19-9) is widely used to assess treatment response and monitor recurrence alongside imaging. However, the criteria for determining resectability after completion of neoadjuvant therapy (NAT) remain poorly defined. Therefore, this study aimed to investigate the indications for surgical resection as a prognostic factor following NAT with gemcitabine and S-1 (NATGS).
Methods: In this retrospective cohort study, we examined patients who underwent curative pancreatic resection following NATGS at our institution between April 2018 and December 2023. After excluding six patients who did not undergo pancreatectomy, the remaining 50 patients were included in the study. Univariate and multivariate analyses were conducted to identify factors potentially associated with survival after NATGS.
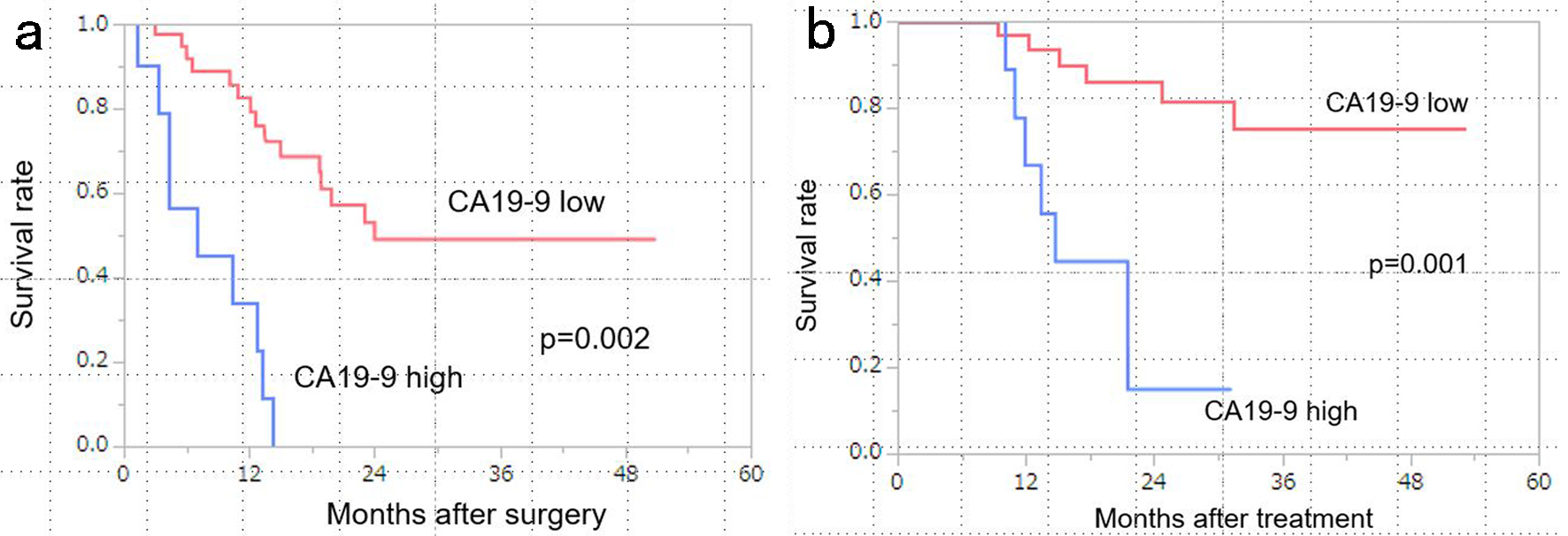
Results: Post-NATGS CA19-9 levels (< 100 U/mL) were identified as a significant prognostic factor for disease-free survival (DFS) in both univariate and multivariate analyses (hazard ratio (HR) = 11.72251, P < 0.001). For overall survival (OS), both CA19-9 levels (< 100 U/mL) and Duke pancreatic monoclonal antigen type 2 (DUPAN-2) levels (< 150 U/mL) were significant prognostic factors in univariate and multivariate analyses (CA19-9: HR = 17.88, P = 0.002; DUPAN-2: HR = 2.667, P = 0.03). The median DFS was 24.1 months in the low CA19-9 group compared with the 7.1 months in the high CA19-9 group (P = 0.002). The median OS in the low CA19-9 group was not reached, whereas it was 14.7 months in the high CA19-9 group (P = 0.001).
Conclusions: The CA19-9 cut-off value is clinically significant for patients undergoing NATGS regimens. Patients with pre-operative CA19-9 levels ≥ 100 U/mL may benefit from extended GS treatment or a switch to a more potent regimen rather than proceeding directly to surgical resection.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









