The Prevalence of 5-Fluorouracil and Capecitabine Cardiotoxicity: A Systematic Review and Meta-Analysis
DOI:
https://doi.org/10.14740/wjon1920Keywords:
5-Fluorouracil, Capecitabine, CardiotoxicityAbstract
Background: The incidence of cardiotoxicity events in patients who use 5-fluorouracil (5-FU) and capecitabine monotherapy remains unclear since previous studies reported the prevalence in patients who used combination regimens. We aimed to systematically review and meta-analyze the incidence of cardiotoxicity in fluorouracil and capecitabine monotherapy users.
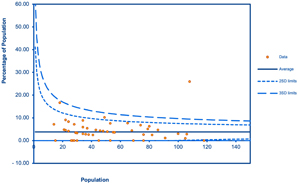
Methods: The study protocol was registered with PROSPERO (CRD42023441627). Systematic searches were conducted in five databases (CINAHL, OpenGrey, PubMed, ScienceDirect, and Scopus). The Cochrane Risk-of-Bias tool and the Risk Of Bias In Non-randomized Studies were used to evaluate the risk of bias. Pooled prevalence and 95% confidence interval (CI) were calculated using the DerSimonian-Laird random effect models. The funnel plot was used to assess the publication bias.
Results: Eighty studies were included. There were 24 randomized controlled trials (RCTs) with low to high risk of bias and 56 non-RCTs with critical risk of bias. The pooled prevalence of cardiotoxicity from 5-FU was 3.5% (95% CI: 2.7 - 4.2; P < 0.001; I2 = 73.86%). The pooled prevalence of cardiotoxicity in capecitabine users was 2.8% (95% CI: 1.6 - 4.0; P < 0.001; I2 = 72.62%).
Conclusions: The prevalence of cardiotoxicity from 5-FU and capecitabine was classified as common. Cardiotoxicity may have not been associated with the cumulative dose of 5-FU or capecitabine.
Published
Issue
Section
License
Copyright (c) 2024 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.