| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 6, December 2025, pages 565-573
Utility of Alpha-Fetoprotein and Protein Induced by Vitamin K Absence or Antagonist-II Kinetics in Predicting Radiologic Response and Survival in Unresectable Hepatocellular Carcinoma Undergoing Immunotherapy
Shou-Wu Leea, b, c, e, Hsin-Ju Tsaia, c, Chia-Chang Chena, c, Yi-Jie Huanga, d, Ying-Cheng Lina, c, Chung-Hsin Changa, Teng-Yu Leea, b, Yen Chun Penga, d
aDivision of Gastroenterology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan, Republic of China
bDepartment of Internal Medicine, Chung Shan Medical University, Taichung, Taiwan, Republic of China
cDepartment of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan, Republic of China
dDepartment of Internal Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan, Republic of China
eCorresponding Author: Shou-Wu Lee, Division of Gastroenterology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung 40705, Taiwan, Republic of China
Manuscript submitted September 15, 2025, accepted September 29, 2025, published online October 31, 2025
Short title: AFP and PIVKA-II Kinetics in HCC Immunotherapy
doi: https://doi.org/10.14740/wjon2679
| Abstract | ▴Top |
Background: Alpha-fetoprotein (AFP) is currently the most commonly used biomarker for hepatocellular carcinoma (HCC) surveillance. However, protein induced by vitamin K absence or antagonist-II (PIVKA-II) may have a better prognostic role in some patients. The aim of this study was to investigate the prognostic roles of AFP and PIVKA-II kinetic changes in predicting outcomes in patients with unresectable HCC receiving immunotherapy.
Methods: Data were collected from subjects with Child-Pugh class A, and Barcelona Clinic Liver Cancer (BCLC) stage B or C HCC, who received immunotherapy from September 2021 to June 2023. The exclusion criteria included cases with normal values of AFP or PIVKA-II. The values of AFP and PIVKA-II at baseline and 4 weeks after initiation of therapy were recorded. The clinical baseline characteristics, and therapeutic outcomes, including radiologic objective response (OR) and overall survival (OS) of enrolled patients were collected and further analyzed.
Results: Among the 33 enrolled patients, 10 and 23 cases achieved OR and non-OR, respectively. A decline in AFP levels of more than 30% from baseline during immunotherapy had the best diagnostic efficacy (0.91) and ideal receiver operating characteristic (ROC) curve (area under the curve (AUC) = 0.907). Combined AFP (≥ 30% decline)/PIVKA (≥ 15% decline) responders had a significantly positive impact on radiologic OR and better OS (hazard ratio (HR): 0.21, 95% confidence interval (CI): 0.06 - 0.73, P = 0.014).
Conclusions: The patients with combined AFP/PIVKA-II decline during immunotherapy had a significantly better radiologic response and survival outcomes.
Keywords: Alpha-fetoprotein; Hepatocellular carcinoma; Immunotherapy; PIVKII
| Introduction | ▴Top |
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and ranks as the leading cause of cancer-related deaths worldwide [1]. The burden of HCC is particularly significant in East Asia and sub-Saharan Africa, largely due to the prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) infections [2]. Despite advancements in diagnosis and treatment, the prognosis of advanced-stage HCC remains dismal due to its aggressive nature and frequent diagnosis at a late stage.
Biomarkers play a pivotal role in early detection, prognostication, and monitoring treatment response of HCC. Alpha-fetoprotein (AFP) has long been the most widely used serum biomarker for HCC surveillance and prognosis [3]. However, the sensitivity and specificity of AFP are suboptimal. Elevated AFP is not always observed in HCC, and it can be falsely elevated in patients with benign liver diseases such as chronic hepatitis or cirrhosis [4]. Moreover, a substantial proportion of HCC patients (30-40%) do not exhibit elevated AFP levels at diagnosis, limiting its utility in both detection and response monitoring [5].
In light of these limitations, other biomarkers have been investigated, with particular interest in protein induced by vitamin K absence or antagonist-II (PIVKA-II), also known as des-gamma-carboxy prothrombin (DCP). PIVKA-II is an abnormal prothrombin molecule produced by malignant hepatocytes due to an acquired defect in the post-translational carboxylation of the prothrombin precursor [6]. Several studies have demonstrated that PIVKA-II is elevated in HCC and correlates with tumor aggressiveness, vascular invasion, and metastatic potential [7, 8]. Besides, its utility in monitoring therapeutic responses and predicting survival outcomes in HCC patients were also provided [9, 10].
The advent of immunotherapy, or called immune checkpoint inhibitors (ICIs), such as anti-programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) and anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) antibodies, has revolutionized the treatment landscape for advanced-stage HCC [11]. However, response rates to ICIs remain modest, and only a subset of patients benefit from immunotherapy, and reliable early biomarkers that predict treatment response are urgently needed. While some data have explored static levels of AFP and PIVKA-II, there is increasing interest in dynamic changes of these markers as predictors of therapeutic response and survival outcomes.
This study aims to evaluate the prognostic value of AFP and PIVKA-II kinetic changes in predicting radiologic responses and overall survival (OS) in patients with unresectable HCC undergoing immunotherapy.
| Materials and Methods | ▴Top |
Study design and patient selection
Figure 1 demonstrates the patient selection process. This retrospective observational cohort study was conducted using clinical data collected between September 2021 and June 2023 at Taichung Veterans General Hospital, a tertiary referral center in Taiwan. The diagnosis of HCC was established based on histological confirmation or noninvasive imaging criteria in accordance with the American Association for the Study of Liver Diseases (AASLD) guidelines [11]. Inclusion criteria were Child-Pugh class A, Barcelona Clinic Liver Cancer (BCLC) stage B or C HCC, receipt of immunotherapy as the first-line treatment, either as monotherapy or in combination with other agents, availability of AFP and PIVKA-II measurements both at baseline and 4 weeks after therapy initiation. Exclusion criteria included normal baseline levels of AFP or PIVKA-II, incomplete follow-up data or missing biomarker measurements, concurrent diagnosis of another malignancy. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committees of Taichung Veterans General Hospital (CE23139B).
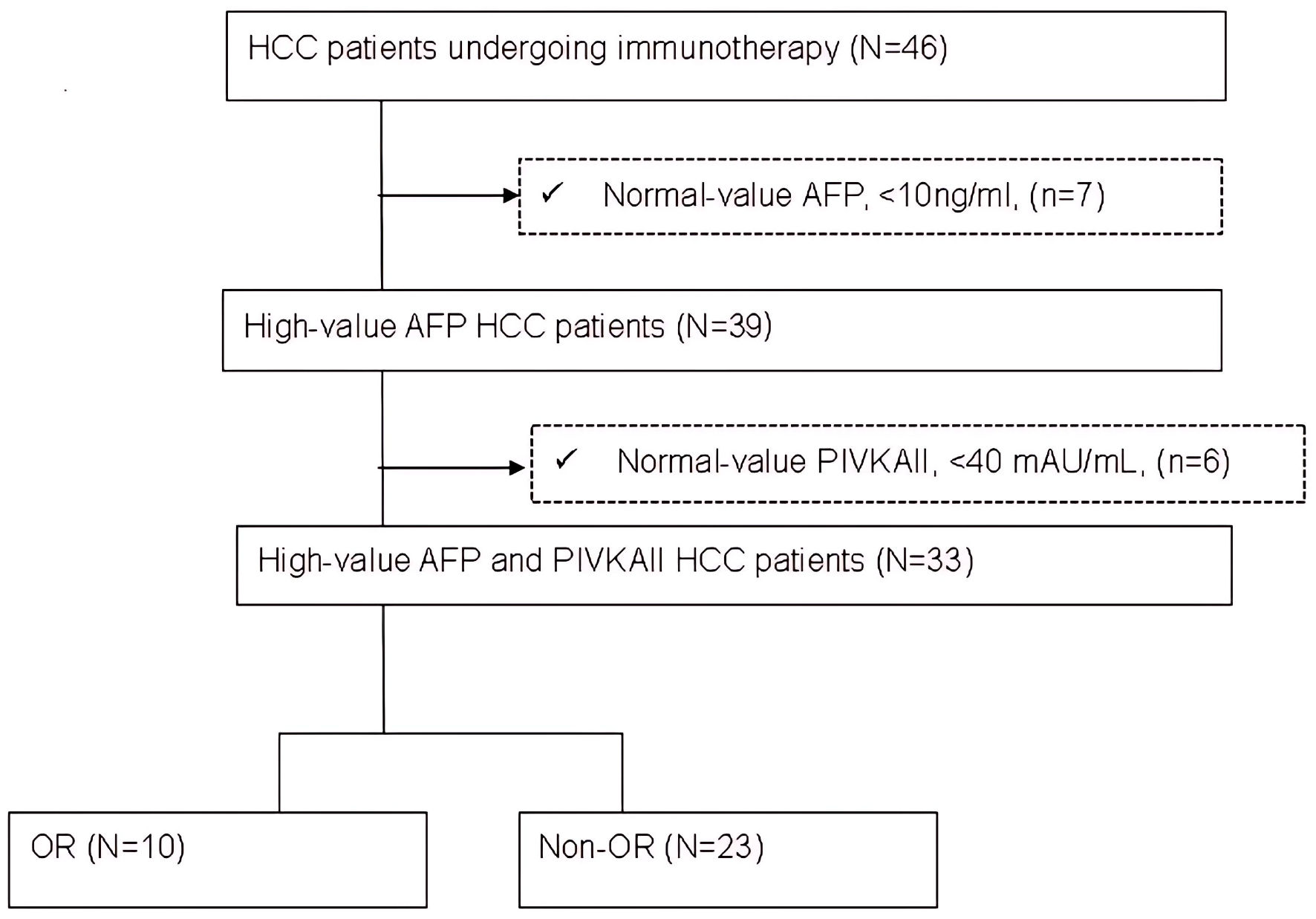 Click for large image | Figure 1. Flow chart of patient selection. AFP: alpha-fetoprotein; HCC: hepatocellular carcinoma; N: number of patients; OR: objective response. |
Baseline characteristics
Clinical, demographic, and treatment-related data were collected from medical records, including age, sex, HBV and HCV status, Albumin-Bilirubin (ALBI) grade, and tumor characteristics, including BCLC stage, macrovascular invasion (MVI), extrahepatic spread (EHS), combinations of other therapy including tyrosine kinase inhibitor (TKI) or locoregional therapy (LRT).
Biomarker measurement and kinetic definitions
Serum AFP and PIVKA-II levels were measured using standardized immunoassays. The cutoff values of AFP > 20 ng/mL or PIVKA-II > 40 mAU/mL were used to define abnormal levels. Kinetic changes in biomarkers were assessed by comparing the values at baseline and 4 weeks post-therapy.
Outcome assessment
The primary outcomes assessed were radiologic objective response (OR), defined by RECIST 1.1 criteria [12] as complete response (CR) or partial response (PR), and OS, defined as the time from initiation of immunotherapy to death from any cause or last follow-up.
Statistical analysis
Continuous variables were reported as mean ± standard deviation (SD) and compared using the independent t-test. Categorical variables such as frequencies and percentages were compared using the Chi-square test or Fisher’s exact test. Receiver operating characteristic (ROC) curve was plotted to determine the diagnostic accuracy of AFP and PIVKA-II kinetics in predicting OR, and the area under the curve (AUC) was used to assess performance. Survival curves were estimated using the Kaplan-Meier method, and differences were compared using the log-rank test. Univariate Cox regression model identified prognostic factors for OS. A P value of < 0.05 was considered statistically significant.
| Results | ▴Top |
Baseline characteristics
A total of 33 patients met the inclusion criteria, and ICI regimens included atezolizumab plus bevacizumab (n = 15), nivolumab (n = 10), or pembrolizumab (n = 8). The baseline characteristics of patients were listed in Table 1. The mean age was 66.4 years, and 78.8% were male. The percentages of HBV and HCV infections were 57.6% and 22.2%, respectively. Distributions by ALBI grade 1 and grade 2 were 45.5% (n = 15) and 54.5% (n = 18), respectively. Regarding HCC BCLC stage, most patients had stage C (n = 29, 87.9%). Tumor characteristics showed that 72.7% had MVI, and 39.4% had EHS, respectively. Nineteen (57.6%) patients had concurrent TKI, and nine (27.3%) patients had concurrent LRT in combination with immunotherapy.
 Click to view | Table 1. Baseline Characteristics in Patients at the Initiation of Immunotherapy |
Radiologic response and biomarker kinetics
As shown in Table 2, of the 33 patients, 10 (30.3%) cases achieved OR, including one CR and nine PR; 23 (69.7%) cases had non-objective response (non-OR), including six SD and 17 PD. There were no significant differences of patients’ baseline characteristics between the cases with OR and those with non-OR.
 Click to view | Table 2. The Best Radiological Tumor Responses of Patients Receiving Immunotherapy |
The diagnostic efficacy of AFP and PIVKA-II decline in predicting the appearance of OR was displayed in Table 3. At 4 weeks after the initiation of immunotherapy, an AFP decline of more than 30% had the highest diagnostic efficacy (0.91; sensitivity 90% and specificity 91%), with an AUC of 0.907. A PIVKA-II decline over 15% showed the highest diagnostic efficacy (0.77; sensitivity 100% and specificity 70%), with an AUC of 0.848. The combination of both biomarkers (AFP decline ≥ 30% + PIVKA-II decline ≥ 15%) had a similar predictive power, with a diagnostic accuracy of 0.91 and an AUC of 0.907. The ROC curve of AFP decline ≥ 30% and PIVKA-II decline ≥ 15% is shown in Figure 2.
 Click to view | Table 3. Diagnostic Efficacy of Biomarker Kinetics at 4 Weeks to Objective Response (OR) of Tumor |
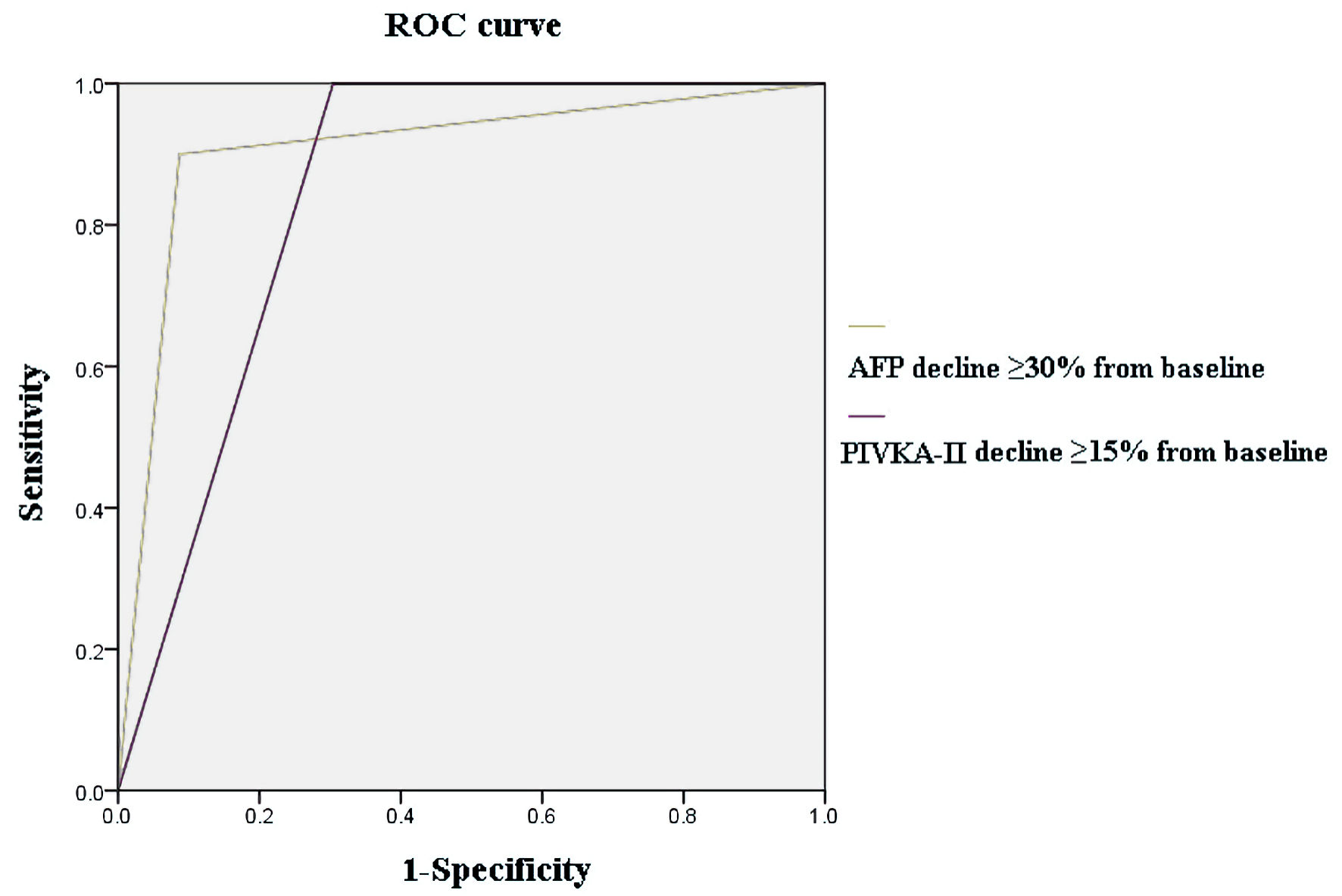 Click for large image | Figure 2. The ROC curve of biomarker kinetics. ROC: receiver operating characteristic; AFP: alpha-fetoprotein. |
Survival analysis
As shown in Table 4, patients’ OS and the associated clinical factors were analyzed. Univariate Cox regression model showed AFP decline ≥ 30% (hazard ratio (HR): 0.32, 95% confidence interval (CI): 0.11 - 0.95, P = 0.041) and combined AFP decline ≥ 30% + PIVKA-II decline ≥ 15% (HR: 0.21, 95% CI: 0.06 - 0.73, P = 0.014) had significant associations with OS.
 Click to view | Table 4. The Strength of Association Between Clinical Parameters and Overall Survival Following Immunotherapy Usage |
The OS of subgroup with different biomarker kinetics was displayed in Figures 3 and 4. Kaplan-Meier curves demonstrated clear separation of survival based on biomarker response. Patients with AFP decline ≥ 30%, or combined AFP and PIVKA-II kinetic response (AFP decline ≥ 30% + PIVKA-II decline ≥ 15%) had significantly better OS compared to non-responders (median OS 15 vs. 4 months, P = 0.011 and 0.004, respectively).
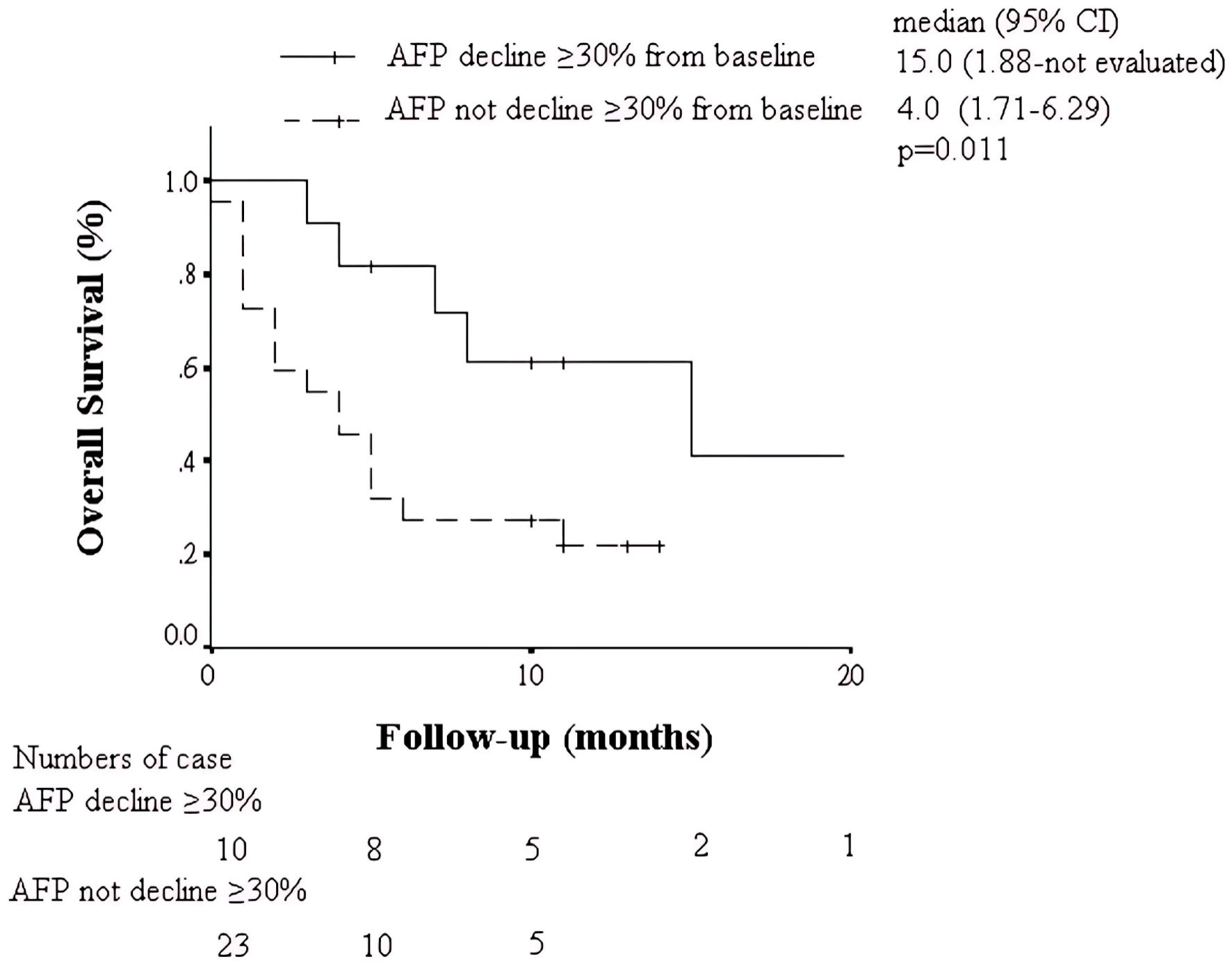 Click for large image | Figure 3. Kaplan-Meier analysis of overall survival in patients with AFP decline ≥ 30% or without. AFP: alpha-fetoprotein; CI: confidence interval. |
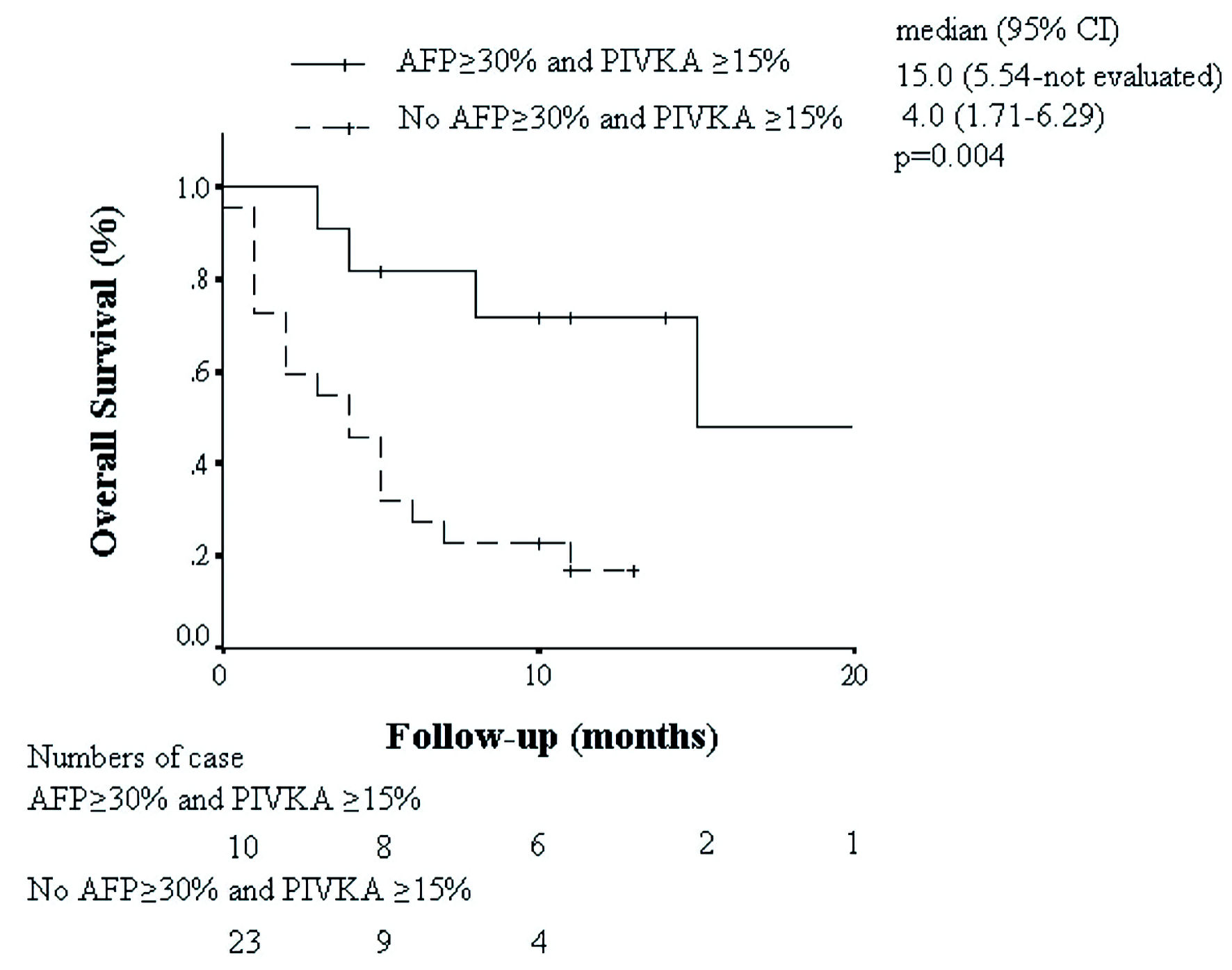 Click for large image | Figure 4. Kaplan-Meier analysis of overall survival in patients with AFP decline ≥ 30% + PIVKA-II decline ≥ 15% or without. AFP: alpha-fetoprotein; CI: confidence interval. |
Furthermore, a stratification of patients according to AFP decline ≥ 30% and PIVKA-II decline ≥ 15% is provided (Supplementary Material 1, wjon.elmerpub.com). In this analysis, one patient exhibited AFP decline without a corresponding PIVKA-II decline, whereas seven patients demonstrated PIVKA-II decline without AFP decline. The table details their baseline characteristics and clinical outcomes, thereby allowing assessment of whether biomarker declines reflect intrinsically more favorable baseline profiles in addition to their association with OS.
| Discussion | ▴Top |
Our findings highlight the prognostic utility of AFP and PIVKA-II kinetic changes in HCC patients receiving immunotherapy. Specifically, a ≥ 30% decrease in AFP from baseline, or combining a ≥ 15% decrease in PIVKA-II from baseline, were strongly associated with improved radiologic response and OS.
AFP is produced by fetal hepatocytes and certain HCC subtypes, particularly poorly differentiated tumors [13]. While AFP has been used as a traditional surveillance tool, its role in tracking response to therapy, particularly immunotherapy, is gaining renewed interest. Several prior studies have shown that a decline in AFP levels during treatment correlates with tumor shrinkage and better survival outcomes [10, 14-18]. Our study confirms this observation and further refines the predictive threshold at 30% decline, aligning with prior reports.
Although AFP decline alone was strongly predictive of OR, the addition of PIVKA-II decline improved OS discrimination without materially changing OR accuracy. This divergence may be explained by the different biological properties of the two markers: AFP reflects tumor burden and dedifferentiation, whereas PIVKA-II is linked to vascular invasion and tumor aggressiveness [19]. Consequently, patients with AFP-only decline may achieve radiologic response but still carry risk for early recurrence or death if PIVKA-II remains elevated. Conversely, PIVKA-II-only responders may have less bulky but biologically aggressive tumors. Thus, combined biomarker kinetics provide more comprehensive prognostication than either marker alone. Furthermore, some reports have suggested that PIVKA-II may be a superior prognostic indicator compared to AFP [8, 20]. In our cohort, a ≥ 15% decline in PIVKA-II combined with AFP kinetics was strongly predictive of OR.
PIVKA-II has been associated with microvascular invasion, tumor progression, and extrahepatic metastasis. Its elevation often reflects aggressive tumor behavior, and its decline may suggest tumor regression or decreased viability.
Unlike chemotherapy or TKI therapy, response to immunotherapy is often delayed and may follow atypical patterns, including pseudoprogression [21]. Therefore, dynamic biomarkers that reflect tumor biology over time can provide crucial guidance. Monitoring early changes (within 4 weeks) can help identify likely responders and non-responders, allowing clinicians to adjust strategies proactively. The combination of AFP and PIVKA-II kinetic responses emerged as a superior prognostic model, likely due to their complementary mechanisms of elevation. While AFP reflects cellular dedifferentiation, PIVKA-II indicates impaired post-translational protein modification. Their combined decline may capture a broader range of therapeutic responses.
Stratification by AFP decline ≥ 30% and PIVKA-II decline ≥ 15% (including one AFP-only and seven PIVKA-II-only cases) provides an overview of baseline characteristics and outcomes across subgroups (Supplementary Material 1, wjon.elmerpub.com). These findings suggest that the prognostic association of biomarker declines may not be entirely explained by baseline differences, although further validation in larger cohorts is warranted.
This study has several limitations. First, the small sample size causes limiting statistical power and generalizability. Second, retrospective design and potential selection bias might exist. Third, biomarker changes were only assessed at one early time point (4 weeks), and additional longitudinal data may provide more robust insights. Despite these limitations, the findings are clinically meaningful and warrant prospective validation in larger, multi-center cohorts. Future research should focus on validating these thresholds in larger trials and exploring the utility of kinetic biomarker profiling in guiding personalized immunotherapy strategies for HCC.
Conclusions
In patients with unresectable HCC receiving immunotherapy, early kinetic declines in AFP (≥ 30%) and combining PIVKA-II (≥ 15%) are significantly associated with objective radiologic response and improved OS. The combination of both biomarkers provides superior prognostic accuracy, supporting their integration into routine clinical monitoring protocols.
| Supplementary Material | ▴Top |
Suppl 1. Baseline characteristics and outcomes by biomarker-decline subgroup.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All other authors have no conflict of interest to disclose.
Informed Consent
This study was approved by the Ethics Committees of Taichung Veterans General Hospital (CE23139B), and the requirement for written informed consent was waived.
Author Contributions
Shou-Wu Lee: project administration, conceptualization, data curation, writing - original draft and approval of final draft. Hsin-Ju Tsai: data curation, data analysis. Chia-Chang Chen: data curation, data analysis. Yi-Jie Huang: data curation, data analysis. Ying-Cheng Lin: data curation, data analysis. Chung-Hsin Chang: data curation, data analysis. Teng-Yu Lee: supervision, manuscript reviewing. Yen-Chun Peng: supervision, manuscript reviewing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263.
doi pubmed - Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34(4):570-575.
doi pubmed - Turshudzhyan A, Wu GY. Persistently rising alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: a review. J Clin Transl Hepatol. 2022;10(1):159-163.
doi pubmed - Lin Y, Ma Y, Chen Y, Huang Y, Lin J, Xiao Z, Cui Z. Diagnostic and prognostic performance of serum GPC3 and PIVKA-II in AFP-negative hepatocellular carcinoma and establishment of nomogram prediction models. BMC Cancer. 2025;25(1):721.
doi pubmed - Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, et al. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310(22):1427-1431.
doi pubmed - Kim DY, Toan BN, Tan CK, Hasan I, Setiawan L, Yu ML, Izumi N, et al. Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region. Clin Mol Hepatol. 2023;29(2):277-292.
doi pubmed - Feng H, Li B, Li Z, Wei Q, Ren L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer. 2021;21(1):401.
doi pubmed - Sagar VM, Herring K, Curbishley S, Hodson J, Fletcher P, Karkhanis S, Mehrzad H, et al. The potential of PIVKA-II as a treatment response biomarker in hepatocellular carcinoma: a prospective United Kingdom cohort study. Oncotarget. 2021;12(24):2338-2350.
doi pubmed - Lee SW, Huang YJ, Lin YC, Tsai HJ, Chen CC, Chang CH, Lee TY, et al. CRAFITY and AFP/PIVKA-II kinetics predict prognosis in hepatocellular carcinoma on immunotherapy. Cancers (Basel). 2025;17(18):3058.
doi pubmed - Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78(6):1922-1965.
doi pubmed - Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
doi pubmed - Johnson PJ. Role of alpha-fetoprotein in the diagnosis and management of hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14(Suppl):S32-36.
doi pubmed - Raoul JL, Park JW, Kang YK, Finn RS, Kim JS, Yeo W, Polite BN, et al. Using Modified RECIST and Alpha-Fetoprotein Levels to Assess Treatment Benefit in Hepatocellular Carcinoma. Liver Cancer. 2014;3(3-4):439-450.
doi pubmed - Tian J, Pan S, Wang Y, Yu Y, Wang S, Shen Y, Yang L, et al. Early alpha-fetoprotein response predicts sustained tumor response following immune checkpoint inhibitors combined with targeted therapy in liver cancer. Biomedicines. 2024;12(12):2769.
doi pubmed - Sun X, Mei J, Lin W, Yang Z, Peng W, Chen J, Zhang Y, et al. Reductions in AFP and PIVKA-II can predict the efficiency of anti-PD-1 immunotherapy in HCC patients. BMC Cancer. 2021;21(1):775.
doi pubmed - Lee PC, Chao Y, Chen MH, Lan KH, Lee CJ, Lee IC, Chen SC, et al. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers (Basel). 2020;12(1):182.
doi pubmed - Hsu WF, Wang HW, Chen CK, Lai HC, Chuang PH, Tsai MH, Su WP, et al. Alpha-fetoprotein response predicts treatment outcomes in patients with unresectable hepatocellular carcinoma receiving immune checkpoint inhibitors with or without tyrosine kinase inhibitors or locoregional therapies. Am J Cancer Res. 2021;11(12):6173-6187.
pubmed - Li T, Yu Y, Liu J, Tian X, Kong M, Wu L, Tang S, et al. PIVKA-II level is correlated to development of portal vein tumor thrombus in patients with HBV-related hepatocellular carcinoma. Infect Agent Cancer. 2019;14:13.
doi pubmed - Guarneri V, Loggi E, Ramacieri G, Serra C, Vukotic R, Vitale G, Scuteri A, et al. Diagnostic performance of PIVKA-II in Italian patients with hepatocellular carcinoma. Cancers (Basel). 2025;17(2):167.
doi pubmed - El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.