| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 6, December 2025, pages 546-554
Reduced Peripheral Blood Natural Killer Cell Proportion Predicts Poor Overall Survival in Advanced Gastric Cancer Patients Treated With Apatinib
Niu Zhanga, d, Si Yu Hua, d, Yu Ting Wanga, d, Hui Shana, Guang Yu Tianb, Yan Wanga, Ye Qing Zhangc, e, Zhi Yuan Qiua, e
aDepartment of Oncology, Affiliated People’s Hospital of Jiangsu University, Zhenjiang, China
bDepartment of Oncology, Jiangdu People’s Hospital Affiliated to Medical College of Yangzhou University, Yangzhou, China
cDepartment of Vascular Surgery, the Second Affiliated Hospital of Soochow University, Suzhou, China
dThese authors contributed equally to this work.
eCorresponding Authors: Ye Qing Zhang, Department of Vascular Surgery, the Second Affiliated Hospital of Soochow University, Suzhou, China; Zhi Yuan Qiu, Department of Oncology, Affiliated People’s Hospital of Jiangsu University, Zhenjiang, China
Manuscript submitted August 25, 2025, accepted October 7, 2025, published online October 17, 2025
Short title: Reduced NK Cell Proportion Predicts Poor OS in AGC Patients
doi: https://doi.org/10.14740/wjon2655
| Abstract | ▴Top |
Background: Angiogenesis inhibition represents a key therapeutic strategy in oncology, with apatinib showing significant efficacy for advanced gastric cancer (AGC) therapy. Currently, no biomarkers can reliably predict which AGC patients will benefit most from apatinib. The purpose of this study was to evaluate apatinib plus tegafur gimeracil oteracil potassium capsule (S-1) as a second-line therapy in AGC and to investigate prognostic biomarkers associated with outcomes.
Methods: All 60 patients received apatinib (250 or 500 mg once daily) in combination with S-1 (40 mg/m2, administered twice daily from day 1 to day 14). Treatment was administered in 28-day cycles, with each course encompassing two such cycles. Multiparameter flow cytometry was used to analyze the subset distribution and immunophenotypes of T cells and natural killer (NK) cells in peripheral blood mononuclear cell samples from AGC patients.
Results: Among all 60 evaluable patients, no complete responses were observed. Partial responses were observed in 33.3% (20/60) of patients (95% confidence interval (CI): 21.1-45.6%), stable disease was observed in 38.3% (23/60) of patients (95% CI: 25.7-51.0%), while progressive disease occurred in 28.3% (95% CI: 16.6-40.1%). Notably, patients with NK cell proportions greater than 17% experienced longer progression-free survival (PFS) and overall survival (OS) than those with lower proportions.
Conclusions: Apatinib combined with S-1 exhibited favorable tolerability and promising clinical activity as a second-line option for AGC patients. Importantly, we identified peripheral blood NK cell proportion as a potential predictor of treatment response, where a low NK cell proportion correlated with poorer outcomes.
Keywords: Apatinib; S-1; Gastric cancer; NK cell
| Introduction | ▴Top |
Gastric cancer (GC) is among the most common malignancies worldwide, ranking fifth in incidence and fourth in cancer-related mortality globally [1, 2]. Although the incidence and mortality of GC have declined in recent years, it continues to rank as the second most prevalent malignancy in China [3, 4]. For GC patients without distant metastasis, surgery is the cornerstone of treatment; however, in China, over 80% of patients are diagnosed at an advanced stage and consequently lose the opportunity for curative surgery [3]. Chemotherapy remains the primary treatment option for advanced gastric cancer (AGC). However, therapeutic options beyond first-line therapy are limited, and nearly all patients eventually experience disease progression. As a second-line strategy, salvage chemotherapy (SLC) has been shown to confer significant survival benefits. In addition, inhibition of angiogenesis has emerged as a promising strategy in cancer treatment.
Apatinib, a small-molecule targeted drug, inhibits vascular endothelial growth factor (VEGF) binding and endothelial cell proliferation. Preliminary data suggest that combining apatinib with chemotherapy shows promising efficacy in AGC patients [4]. To improve the survival of AGC patients, treatment strategies must be optimized, with biomarker application playing a crucial role in guiding therapeutic decisions. However, reliable biomarkers to identify AGC patients who may derive benefit from apatinib remain unavailable. Consequently, identifying new prognostic biomarkers that can predict treatment response has become an urgent priority. Notably, the immune system is key to the development and progression of AGC. Clinical research examining the immune profile of AGC patients has highlighted its potential influence on therapeutic outcomes. Functional analyses further suggest that variations in immune competence may modulate treatment responsiveness. Given the influence of the immune system on treatment response, certain immune cell populations - like T cells and natural killer (NK) cells - have attracted considerable attention in clinical biomarker research. Several immune cell populations, reported to be associated with favorable prognoses, have been proposed as potential predictors of prognosis and as indicators for evaluating therapeutic outcomes in clinical practice [5-8].
In this study, the primary aim was to evaluate the prognostic significance of circulating T cells and NK cells in AGC patients using multiparameter flow cytometry (FCM), and to determine whether these subsets could independently predict progression-free survival (PFS) and overall survival (OS). The secondary aim was to assess the effectiveness of apatinib plus tegafur gimeracil oteracil potassium capsule (S-1) as a second-line therapy for GC.
| Materials and Methods | ▴Top |
Patient eligibility and clinical characteristics
Between December 2015 and December 2020, 60 AGC patients who had failed first-line chemotherapy were enrolled and followed up retrospectively at the Department of Oncology, Affiliated People’s Hospital of Jiangsu University. The inclusion criteria included: 1) age between 18 and 80 years; 2) histologically confirmed GC; 3) presence of at least one measurable lesion; 4) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 - 2; 5) sufficient organ function confirmed by laboratory parameters; and 6) lymphocyte subsets were examined before treatment. The exclusion criteria included: 1) pregnancy or breastfeeding; 2) having a current or previous diagnosis of another malignancy; and 3) patients with central nervous system (CNS) metastases were excluded because their prognosis and treatment response differ from patients without CNS involvement, which could confound the analysis. Clinical characteristics, including age, sex, ECOG PS, lymph node status, and complete blood count (CBC) results, were obtained from medical records. Computed tomography (CT) scans were conducted to assess staging and evaluate treatment efficacy at diagnosis.
Immunophenotypic analysis
Peripheral blood (PB) samples were obtained from all patients before starting treatment. Additionally, peripheral blood mononuclear cells (PBMCs) from a healthy donor served as an internal control. FCM analysis was conducted to assess the proportions of circulating lymphocyte subsets in PB, including CD3+CD4+ T cells, CD3+CD8+ T cells, and NK cells (CD3-CD16+CD56+). These analyses were conducted with a Becton-Dickinson flow cytometer (San Jose, CA). The following murine anti-human monoclonal antibodies (BD Biosciences) were employed: CD16-PE (clone 3G8), CD8-APC (clone SK1), CD4-PE (clone RPA-T4), CD3-FITC (clone SK7), and CD56-APC (clone NCAM16.2). Isotype-matched IgG1 antibodies served as negative controls to minimize nonspecific staining. The experimental procedures for assessing cellular immunological indicators were conducted as previously described [9]. Briefly, mature erythrocytes were lysed using a Tris-NH4Cl solution. Fresh PB samples were then incubated with a fluorescent antibody cocktail for 15 min at room temperature (20 - 25 °C) in the dark. Data acquisition and analysis were performed using CellQuest software (BD Biosciences).
Treatment and assessment
Apatinib and S-1 were provided by Jiangsu Hengrui Medicine Co., Ltd. Patients were treated with apatinib (250 or 500 mg once daily) and S-1 (40 mg/m2 twice daily) on days 1 - 14. Each treatment cycle lasted 28 days, with two cycles constituting one course. Therapy continued until disease progression, intolerable toxicity, or another reason for discontinuation. Dose reductions or treatment delays were implemented in the event of significant adverse events (AEs).
Every 8 weeks, treatment efficacy was determined in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Complete responses (CRs) and partial responses (PRs) were verified by sequential CT scans obtained at an interval of 4 weeks or longer. Evaluation of AEs was performed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Follow-up and statistical analysis
Statistical analyses were performed with SPSS software, version 23.0 (SPSS Inc., Chicago, IL). Variables included in the analysis comprised age, gender, histology, apatinib dose intensity, and the proportions of circulating lymphocyte subsets proportions (CD4+, CD8+ T cells, and NK cells). Student’s t-test or corresponding nonparametric tests were applied for continuous variables, and Pearson’s Chi-square or Fisher’s exact test was used for categorical data, as appropriate. Receiver operating characteristic (ROC) analysis was used to establish cutoff values. The primary endpoints comprised PFS and OS, while secondary endpoints encompassed objective response rate (ORR), disease control rate (DCR), safety, and potential biomarkers. PFS was measured from the beginning of therapy to either disease progression or death, and OS from treatment initiation to death or the end of follow-up. ORR was defined as the proportion of patients achieving a best overall response of either a CR or a PR, confirmed by a subsequent assessment at least 4 weeks later. DCR was defined as the proportion of patients achieving a best overall response of CR, PR, or stable disease (SD). Kaplan-Meier analysis with log-rank tests was employed to compare OS and PFS, and Cox proportional hazards regression was used for univariate and multivariate analyses. P-values < 0.05 (two-tailed) were considered statistically significant.
Ethical statements
The study was approved by the Ethics Committee of the Affiliated People’s Hospital of Jiangsu University and conducted in accordance with the Declaration of Helsinki.
| Results | ▴Top |
Characteristics of enrolled patients
Sixty patients with AGC participated in the study, consisting of 36 men (60.0%) and 24 women (40.0%), with a median age of 64.6 ± 8.3 years (range, 30 to 80 years). All patients exhibited favorable ECOG PS, with 45 (75.0%) at PS 0, 10 (16.7%) at PS 1, and five (8.3%) at PS 2. Detailed patient characteristics are presented in Table 1. Among the 60 patients, 38 (63.3%) had undergone prior surgery, and all had received first-line chemotherapy. Metastases were most frequently observed in abdominal lymph nodes (78.3%), with the peritoneum (53.3%), liver (43.3%), and lungs (26.7%) also commonly involved. The initial dose of apatinib was 500 mg in 24 patients and 250 mg in 36 patients. The first-line chemotherapy regimens included S-1 and oxaliplatin (SOX) in 66.7% of patients, docetaxel plus S-1 in 15%, and a fluoropyrimidine-oxaliplatin combination in the remaining 18.3% (Table 1).
 Click to view | Table 1. Characteristics of 60 AGC Patients |
Efficacy
Treatment response was evaluable in all 60 patients. No CRs were observed; 20 patients (33.3%, 95% CI: 21.1-45.6%) achieved PRs. SD occurred in 23 patients (38.3%, 95% CI: 25.7-51.0%), while progressive disease (PD) was noted in 17 patients (28.3%, 95% CI: 16.6-40.1%). The ORR was 33.3% (95% CI: 21.1-45.6%) and the DCR was 71.7% (95% CI: 59.9-83.4%). In the study population, the median PFS was 175.8 ± 12.6 days (95% CI: 151.1 - 200.5) and the median OS was 280.4 ± 18.8 days (95% CI: 243.5 - 317.2) (Fig. 1).
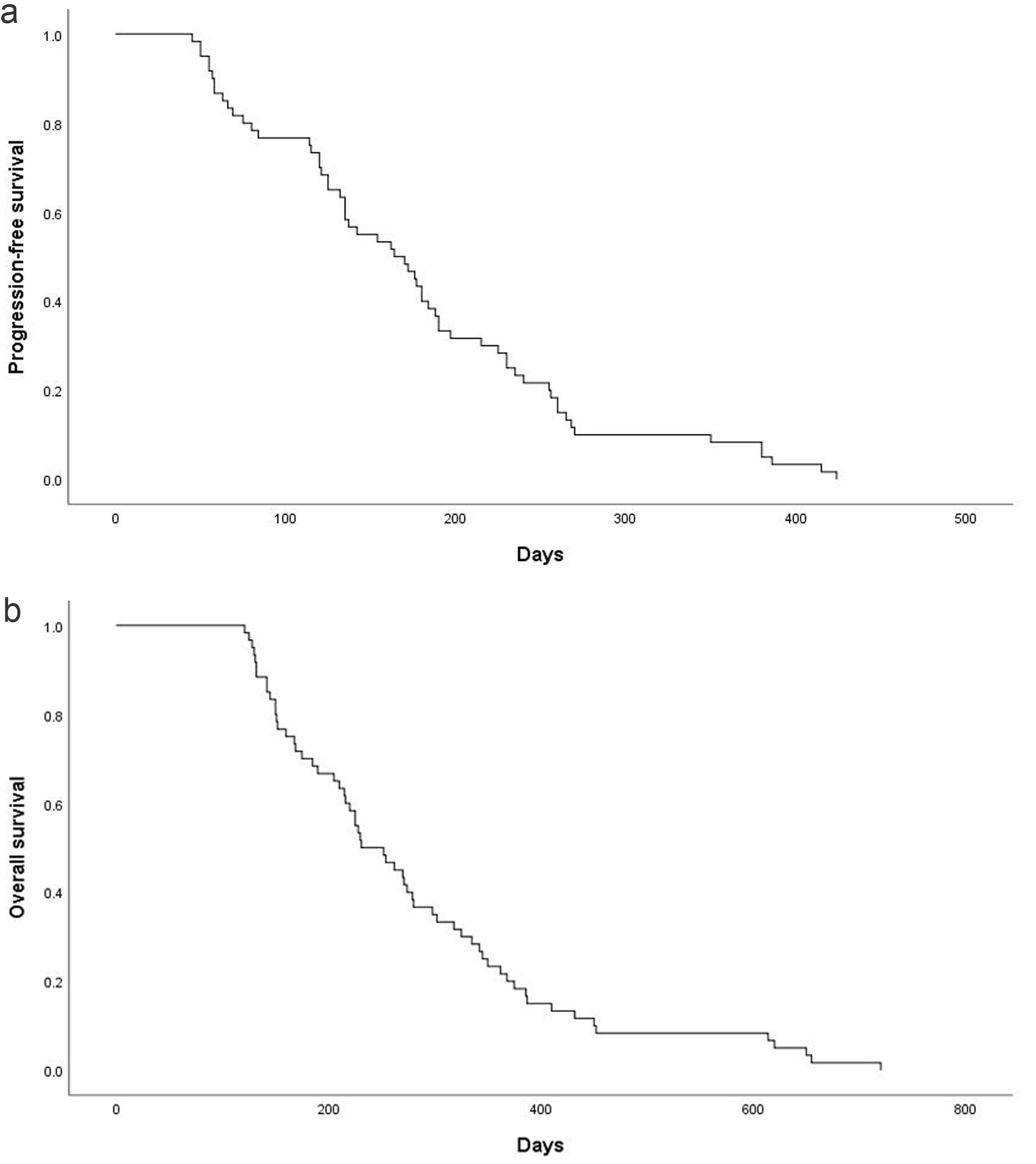 Click for large image | Figure 1. Kaplan-Meier estimates of PFS and OS. (a) PFS for the overall population and the median PFS was 175.8 ± 12.6 days (95% CI: 151.1 - 200.5). (b) OS for the overall population and the median OS was 280.4 ± 18.8 days (95% CI: 243.5 - 317.2). CI: confidence interval; OS: overall survival; PFS: progression-free survival. |
Safety
Toxicity was assessed in all patients (n = 60), and the regimen was generally well tolerated. Neutropenia, anemia, and thrombocytopenia were observed in 50.0%, 10.0%, and 18.3% of patients, respectively. The most frequent non-hematological toxicities included fatigue (50.7%), hypertension (48.3%), hand-foot syndrome (23.3%), proteinuria (13.3%), and diarrhea (10.0%) (Table 2). Overall, non-hematological toxicities were predominantly mild to moderate, with severe events occurring infrequently.
 Click to view | Table 2. Incidence of AEs During the Treatment |
Prognostic significance of NK cell proportion in GC patients
To assess the prognostic value of NK cells in AGC patients, time-dependent ROC analysis was performed and the area under the curve (AUC) was calculated. Using SPSS, the ROC curve was analyzed to establish the optimal cutoff, resulting in an AUC of 0.863 (95% CI: 0.766 - 0.960) (Fig. 2). The optimal cutoff for the percentage of NK cells was determined to be 17%, based on the maximum Youden index of approximately 0.577. At this threshold, the corresponding sensitivity and specificity were approximately 93% and 64.7%, respectively.
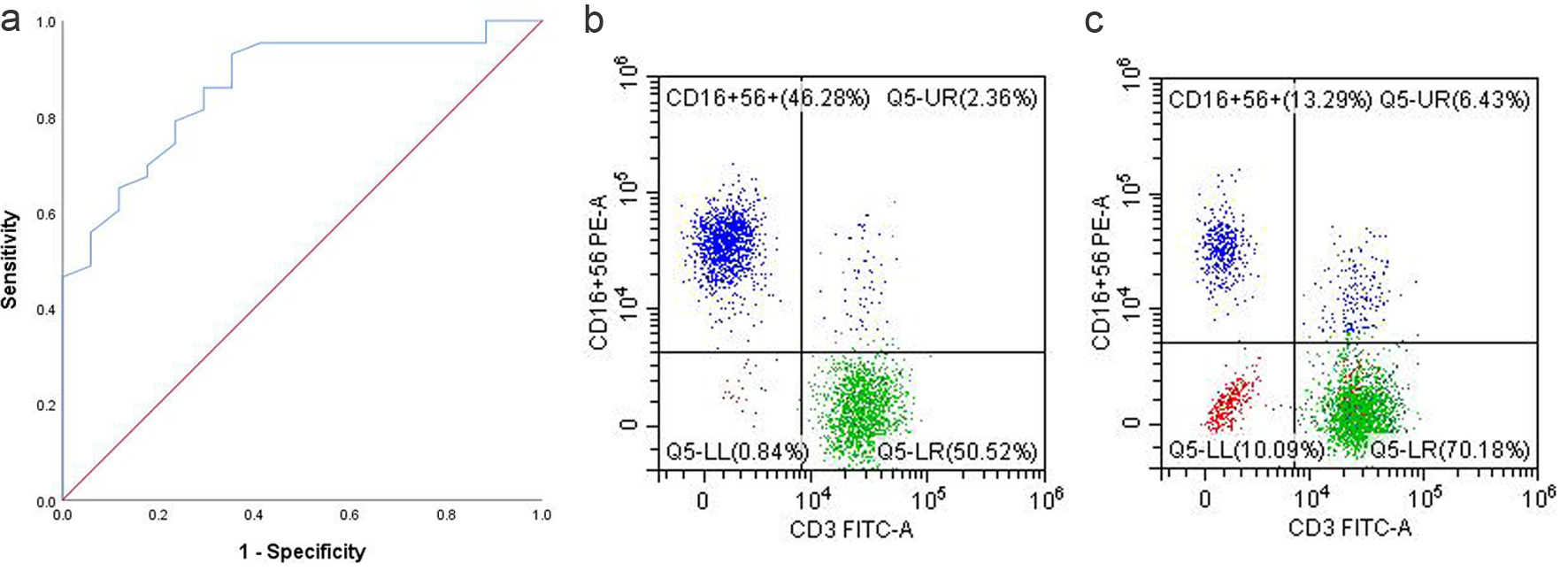 Click for large image | Figure 2. (a) ROC curve of NK cell proportion. (b) NK cell proportion in the PB of responders. (c) NK cell proportion in the PB of non-responders. NK: natural killer; PB: peripheral blood; ROC: receiver operating characteristic. |
Patients were divided into proportion of NK cells ≥ 17% (n = 39) and proportion of NK cells < 17% (n = 21). Following dichotomization using the optimal cutoff value of 17%, outcome analysis revealed significant differences between the two subgroups. Patients with a high NK cell proportion (> 17%) had longer PFS than those with a low proportion, with median PFS of 210.5 ± 15.3 days versus 111.4 ± 14.1 days (P < 0.001; Fig. 3a). Similarly, OS was longer in patients with a high NK cell proportion than in those with a low proportion, with a median OS of 329.4 ± 24.0 days versus 189.3 ± 18.7 days (P < 0.001; Fig. 3b).
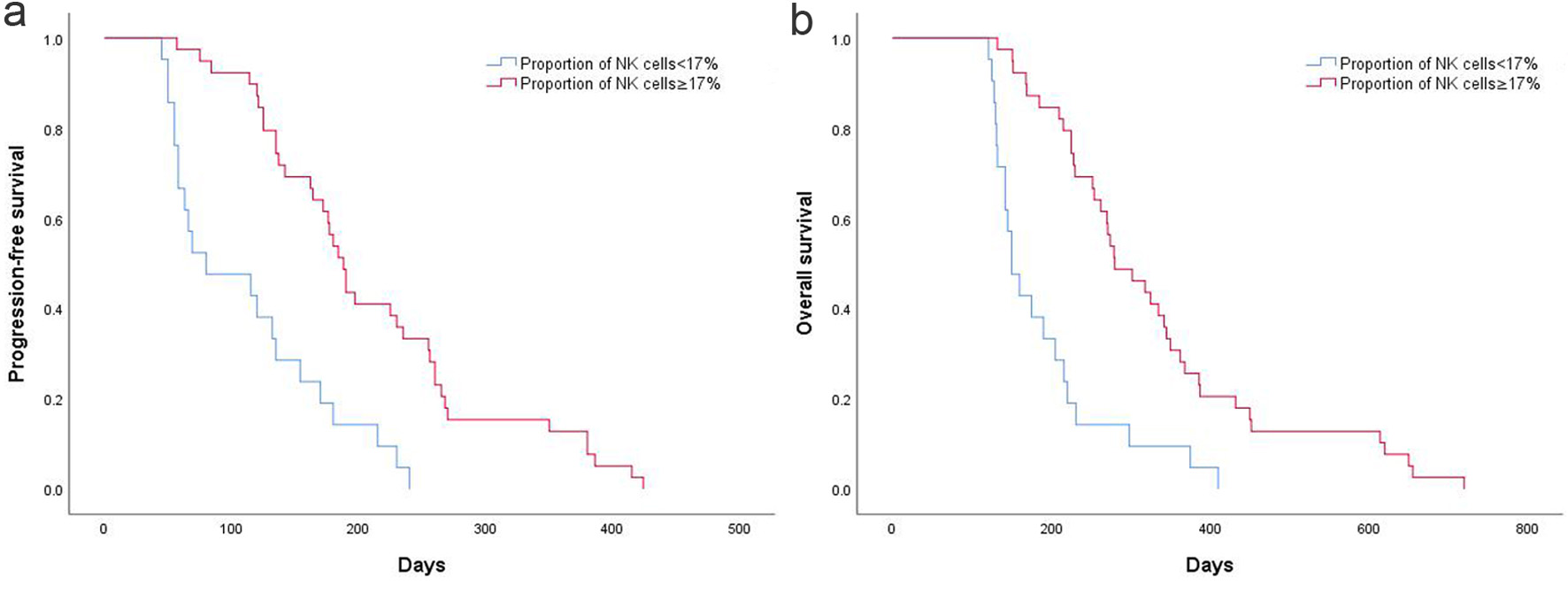 Click for large image | Figure 3. (a) High and low NK cell proportion on PFS in AGC patients. (b) High and low NK cell proportion on OS in AGC patients. AGC: advanced gastric cancer; NK: natural killer; OS: overall survival; PFS: progression-free survival. |
Prognostic significance of T-cell proportion in GC patients
To assess the clinical significance of CD8+ T cells on survival in patients with AGC, the AUC was calculated and found to be 0.828 (95% CI: 0.718 - 0.937) (Fig. 4). The optimal cutoff for the percentage of CD8+ T cells was determined to be 25.5%, based on the maximum Youden index, which was approximately 0.685. At this cutoff point, the corresponding sensitivity was approximately 94.1%, and the specificity was approximately 74.4%.
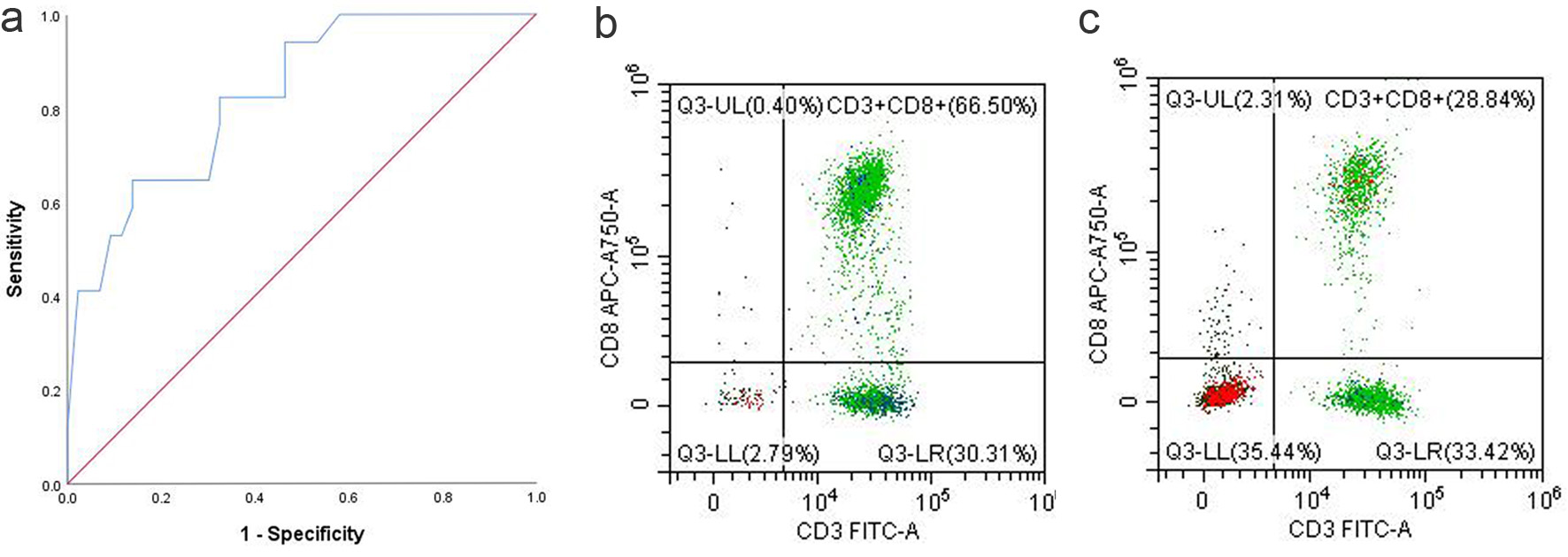 Click for large image | Figure 4. (a) ROC curve of CD8+ T-cell proportion. (b) CD8+ T proportion in the PB of responders. (c) CD8+ T proportion in the PB of non-responders. PB: peripheral blood; ROC: receiver operating characteristic. |
Patients were divided into CD8+ T cell ≥ 25.5% (n = 28) and CD8+ T cell < 25.5% (n = 32). Following dichotomization using the optimal cutoff value of 25.5%, PFS and OS did not differ significantly between the high- and low-level groups. Patients with a low CD8+ T-cell proportion had a median PFS of 164.0 ± 19.9 days, compared with 186.1 ± 16.1 days in the high-proportion group (P = 0.45; Fig. 5a). Median OS was 269.9 ± 28.8 days for patients with a low CD8+ T-cell proportion and 289.5 ± 25.0 days for those with a high proportion (P = 0.84; Fig. 5b). After dividing patients by the 25.5% cutoff, no significant differences in PFS or OS were observed between the groups. Patients with a low CD8+ T-cell proportion had a median PFS of 164.0 ± 19.9 days, compared with 186.1 ± 16.1 days in those with a high proportion (P = 0.45; Fig. 5a). Patients with a low CD8+ T-cell proportion had a median OS of 269.9 ± 28.8 days, compared with 289.5 ± 25.0 days in those with a high proportion (P = 0.84; Fig. 5b).
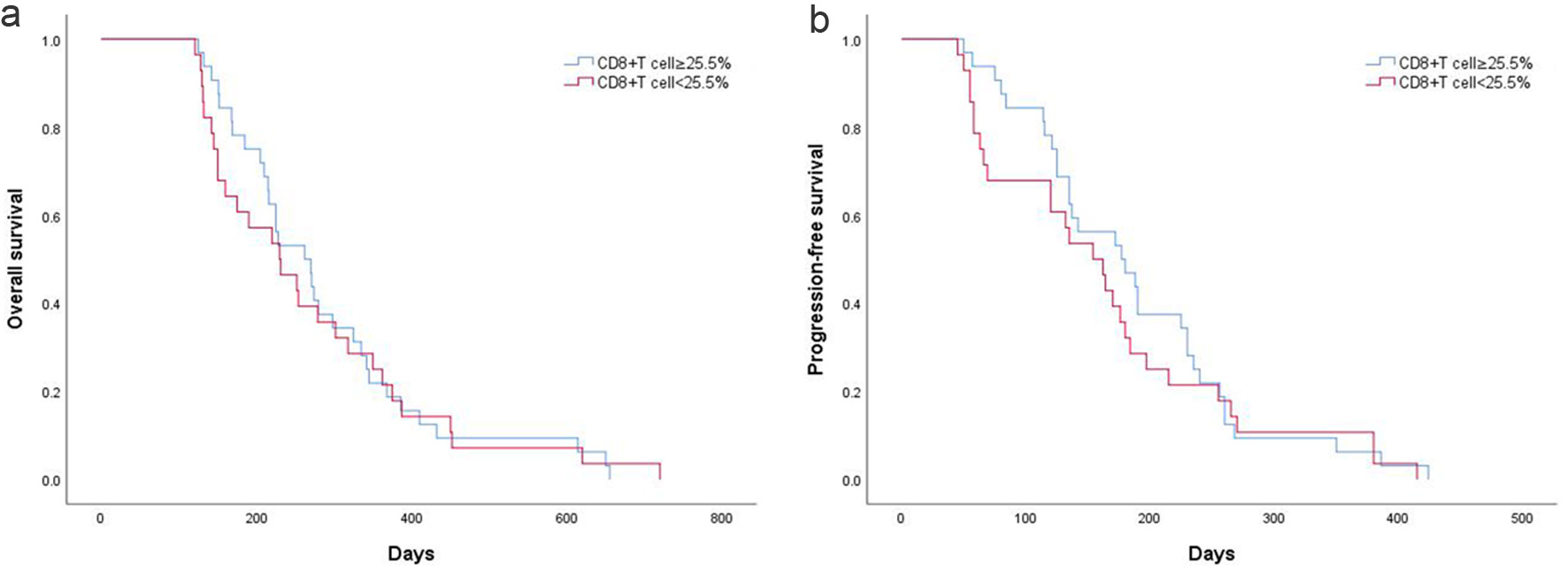 Click for large image | Figure 5. (a) PFS according to CD8+ T-cell proportion in AGC patients. (b) OS according to CD8+ T-cell proportion in AGC patients. AGC: advanced gastric cancer; OS: overall survival; PFS: progression-free survival. |
Cox regression analyses were performed to evaluate clinical characteristics, including gender, age, metastatic sites, CD8+ T-cell proportion, and NK cell proportion. Univariate analysis revealed that ECOG PS was a significant predictor of both PFS and OS (PFS: hazard ratio (HR) = 3.281, 95% CI: 2.14 - 5.04, P < 0.001; OS: HR = 3.748, 95% CI: 2.37 - 5.92, P < 0.001). Multivariate analysis indicated that ECOG PS and NK cell proportion were independent predictors of PFS and OS in AGC patients. Conversely, age, gender, histology, apatinib dose intensity, and metastatic sites were not identified as significant predictors of survival outcomes.
| Discussion | ▴Top |
To date, the role of systemic immune profiles in tumor progression remains incompletely understood. We investigated the immunophenotypic characteristics of peripheral T and NK cells in AGC and analyzed their correlation with patients’ clinical prognosis. Understanding how lymphocyte subsets respond to therapeutic interventions is essential for the potential use of PB immune cell profiles as biomarkers for patient monitoring. Clinical evidence indicates that combining anti-VEGF antibodies with chemotherapy can markedly prolong PFS in GC patients [10]. Therefore, we retrospectively analyzed the safety and efficacy of apatinib plus S-1 in AGC patients whose disease progressed following first-line chemotherapy. We further explored whether circulating T and NK cells in PB could serve as biomarkers linked to clinical outcomes.
In this study, the median PFS and OS were 175.8 and 280.4 days, respectively, which seem to surpass those reported in earlier studies. In the study by Zhao et al, AGC patients treated with the combination of apatinib and paclitaxel-based chemotherapy showed a median PFS of 4.21 months and OS of 7.49 months [11]. Furthermore, the median PFS and OS in our cohort were similar to those observed in the RAINBOW study, which assessed ramucirumab combined with paclitaxel as second-line therapy for AGC (median PFS, 4.4 months; median OS, 9.6 months) [12]. Thus, our findings suggest favorable efficacy and a manageable safety profile in Chinese AGC patients treated in real-world settings. The combination of apatinib and S-1 targets the VEGF pathway, a well-established and critical therapeutic target in cancer treatment. VEGF promotes the expansion of immunosuppressive cells, including regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), while suppressing the differentiation of progenitor cells into CD4+ and CD8+ T lymphocytes. As a result, T-cell expansion and cytotoxic activity are impaired. Furthermore, VEGF contributes to the exhaustion of T cells via the upregulation of checkpoint molecules like programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), T-cell immunoglobulin mucin-3 (TIM-3), and lymphocyte-activation gene 3 (LAG-3). Together, these mechanisms provide a strong mechanistic rationale for the apatinib/S1 combination therapy.
Recent research has emphasized the prognostic and predictive significance of certain immune cell subsets [13-15]. These immune cell subsets, representing both innate and adaptive branches, consist of T lymphocytes, NK cells, and dendritic cells. The immune status of the host can substantially influence treatment efficacy through multiple mechanisms. Lymphocytes contribute critically to both innate and adaptive antitumor immunity and can be divided into distinct functional subsets [13, 14]. NK cells, commonly characterized by CD16 and CD56 surface expression, exhibit potent cytotoxic activity and contribute synergistically to antitumor responses [15]. CD8+ T cells target tumor antigens through classical HLA class I molecules, while CD4+ T cells exert crucial regulatory functions by secreting cytokines and activating both B cells and cytotoxic T cells.
Similar to how circulating non-coding RNA and DNA in PB reflect certain tumor characteristics [16], lymphocytes in PB have been shown to relate to the immune microenvironment and lymphocyte infiltration within tumor tissues. In certain malignancies, elevated levels of cytotoxic lymphocytes in PB often indicate high levels of immune infiltration within the tumor, which are associated with favorable treatment outcomes; whereas enrichment of immunosuppressive cells is frequently associated with poor treatment outcomes and prognosis [17, 18].
GC exhibits a highly heterogeneous clinical course, with the immune system critically shaping the tumor microenvironment and modulating patient outcomes in a treatment-dependent manner [5]. Several studies have suggested that a higher absolute lymphocyte count (ALC) correlates with improved OS in follicular lymphoma (FL) patients [7]; however, these associations remain controversial. Currently, no validated predictive biomarkers exist for apatinib, and data regarding NK cells in the PB of GC patients are limited. In our research, we retrospectively analyzed the distribution and immunophenotypes of circulating T cells and NK cells in GC patients, quantifying their proportions to inform treatment selection. Building on prior findings, we focused on immune factors relevant to the pharmacological mechanism underlying apatinib’s therapeutic effects. Specifically, NK cells and T cells were assessed for their prognostic significance and potential predictive value for apatinib efficacy.
Our findings suggest that a low NK proportion in the PB may be associated with poorer outcomes in GC, whereas no significant correlation was observed between T cells and survival. Furthermore, multivariate analysis identified NK cells proportion as an independent predictor of both PFS and OS. Previous studies have shown that elevated NK cell counts correlate with more favorable clinical outcomes in patients with diffuse large B-cell lymphoma (DLBCL) [19], a reduced lymphocyte-to-monocyte ratio serves as a prognostic indicator of unfavorable outcomes in DLBCL patients [20], and the ratios of NK and T cells to tumor cells independently forecast the time to treatment initiation in patients with chronic lymphocytic leukemia (CLL) [21].
To date, no data have linked specific populations of circulating lymphocytes to survival outcomes in GC, and the mechanisms underlying these potential associations remain largely unknown. NK cells, which can directly target and kill tumor cells without prior sensitization, are categorized into two subsets based on the surface markers CD16 and CD56. CD16+CD56dim NK cells, which account for approximately 90% of PB NK cells, are rich in intracellular perforin, and exhibit potent cytotoxic activity [15]. Emerging evidence also indicates that, in addition to their direct cytolytic effects, NK cells can influence the adaptive immune response by fostering a T helper 1 (Th1) profile, which supports antitumor immunity [15].
However, this study has several limitations that should be acknowledged. First, it was a retrospective, single-center analysis with a relatively small sample size, which may limit the generalizability of our findings. Second, although we observed correlations between treatment outcomes and NK cell activity, functional assays were not performed to directly confirm the mechanistic role of NK cells. Third, heterogeneity in apatinib dosing among patients may have influenced treatment outcomes. Finally, the potential impact of prior treatment regimens could not be fully excluded, as patients had received different therapies before enrollment. These limitations should be taken into consideration when interpreting our results, and future large-scale, prospective studies incorporating mechanistic investigations are warranted to validate and extend our findings.
In conclusion, our study demonstrated that apatinib in combination with S1 was well tolerated, with promising efficacy data observed in the second-line treatment of AGC. Additionally, biomarker analysis suggested that the NK proportion could serve as a prognostic indicator for OS in GC, providing valuable insights for outcome assessment. Our study highlights potential candidate biomarkers that may aid in identifying patients who are most likely to respond to apatinib therapy. Our findings indicate that apatinib’s efficacy may be improved in patients with a higher NK proportion. Furthermore, immune cells are likely to play a crucial role in disease progression and represent promising therapeutic targets in GC.
Acknowledgments
The authors wish to thank all patients and their families for participating in this study, as well as the clinical and laboratory staff at the Department of Oncology, Affiliated People’s Hospital of Jiangsu University, for their invaluable support and contributions.
Financial Disclosure
This work was supported by Medical Research Project of Yangzhou Health Commission 2023 (2023-2-28), Social Development Foundation of Zhenjiang (SH2019069), and Science Foundation of the Affiliated People’s Hospital of Jiangsu University (Y2019021-S).
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Written informed consent was obtained from all participants prior to enrollment.
Author Contributions
Zhi Yuan Qiu conceived and designed the study, Si Yu Hu and Yu Ting Wang researched the literature, Zhi Yuan Qiu and Hui Shan wrote the manuscript, Yan Wang and Guang Yu Tian designed the figures, Niu Zhang and Ye Qing Zhang revised and edited the article. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33.
doi pubmed - Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Chen W, Sun K, Zheng R, Zeng H, Zhang S, Xia C, Yang Z, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30(1):1-12.
doi pubmed - Zhang Y, Xu J, Wang Q, Ling G, Mao Y, Cai M, Yang Y, et al. Efficacy and safety of second-line therapy with apatinib combined with chemotherapy as second-line therapy in advanced gastric cancer: a single-arm, open-label, prospective, multicenter study. Ann Transl Med. 2022;10(11):641.
doi pubmed - Zhou XH, Zhang XY, Liang JH, Zhu HY, Wang L, Xia Y, Cao L, et al. Low absolute NK cell counts in peripheral blood are associated with inferior survival in patients with mantle cell lymphoma. Cancer Biomark. 2019;24(4):439-447.
doi pubmed - Zhang YP, Zhang R, Zhu HY, Wang L, Wu YJ, Liang JH, Shi WY, et al. Circulating low absolute CD4+ T cell counts may predict poor prognosis in extranodal NK/T-cell lymphoma patients treating with pegaspargase-based chemotherapy. Cancer Res Treat. 2019;51(1):368-377.
doi pubmed - He L, Zhu HY, Qin SC, Li Y, Miao Y, Liang JH, Xia Y, et al. Low natural killer (NK) cell counts in peripheral blood adversely affect clinical outcome of patients with follicular lymphoma. Blood Cancer J. 2016;6(8):e457.
doi pubmed - Shafer D, Smith MR, Borghaei H, Millenson MM, Li T, Litwin S, Anad R, et al. Low NK cell counts in peripheral blood are associated with inferior overall survival in patients with follicular lymphoma. Leuk Res. 2013;37(10):1213-1215.
doi pubmed - Qiu ZY, Shen WY, Fan L, Wang L, Yu H, Qiao C, Wu YJ, et al. Assessment of clonality in T-cell large granular lymphocytic leukemia: flow cytometric T cell receptor Vbeta repertoire and T cell receptor gene rearrangement. Leuk Lymphoma. 2015;56(2):324-331.
doi pubmed - Kang Y, Ohtsu A, Van Cutsem E, Rha SY, Sawaki A, Park S, Lim H, et al. AVAGAST: A randomized, double-blind, placebo-controlled, phase III study of first-line capecitabine and cisplatin plus bevacizumab or placebo in patients with advanced gastric cancer (AGC). J Clin Oncol. 2010;28(Suppl 18):LBA4007.
doi - Zhao S, Fan N, Li H, Liu J, Huang F, Chen Y, Zhou M, et al. Apatinib combined with paclitaxel-based chemotherapy in patients with taxane-resistant advanced gastric cancer: a single-arm exploratory study. Ann Transl Med. 2020;8(19):1233.
doi pubmed - Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224-1235.
doi pubmed - Fan X, Rudensky AY. Hallmarks of tissue-resident lymphocytes. Cell. 2016;164(6):1198-1211.
doi pubmed - Grossman Z, Paul WE. Dynamic tuning of lymphocytes: physiological basis, mechanisms, and function. Annu Rev Immunol. 2015;33:677-713.
doi pubmed - Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19(1):120.
doi pubmed - Alix-Panabieres C, Pantel K. Liquid biopsy: from discovery to clinical application. Cancer Discov. 2021;11(4):858-873.
doi pubmed - van der Leun AM, Thommen DS, Schumacher TN. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. 2020;20(4):218-232.
doi pubmed - Afghahi A, Purington N, Han SS, Desai M, Pierson E, Mathur MB, Seto T, et al. Higher Absolute Lymphocyte Counts Predict Lower Mortality from Early-Stage Triple-Negative Breast Cancer. Clin Cancer Res. 2018;24(12):2851-2858.
doi pubmed - Plonquet A, Haioun C, Jais JP, Debard AL, Salles G, Bene MC, Feugier P, et al. Peripheral blood natural killer cell count is associated with clinical outcome in patients with aaIPI 2-3 diffuse large B-cell lymphoma. Ann Oncol. 2007;18(7):1209-1215.
doi pubmed - Li ZM, Huang JJ, Xia Y, Sun J, Huang Y, Wang Y, Zhu YJ, et al. Blood lymphocyte-to-monocyte ratio identifies high-risk patients in diffuse large B-cell lymphoma treated with R-CHOP. PLoS One. 2012;7(7):e41658.
doi pubmed - Palmer S, Hanson CA, Zent CS, Porrata LF, Laplant B, Geyer SM, Markovic SN, et al. Prognostic importance of T and NK-cells in a consecutive series of newly diagnosed patients with chronic lymphocytic leukaemia. Br J Haematol. 2008;141(5):607-614.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.