Figures
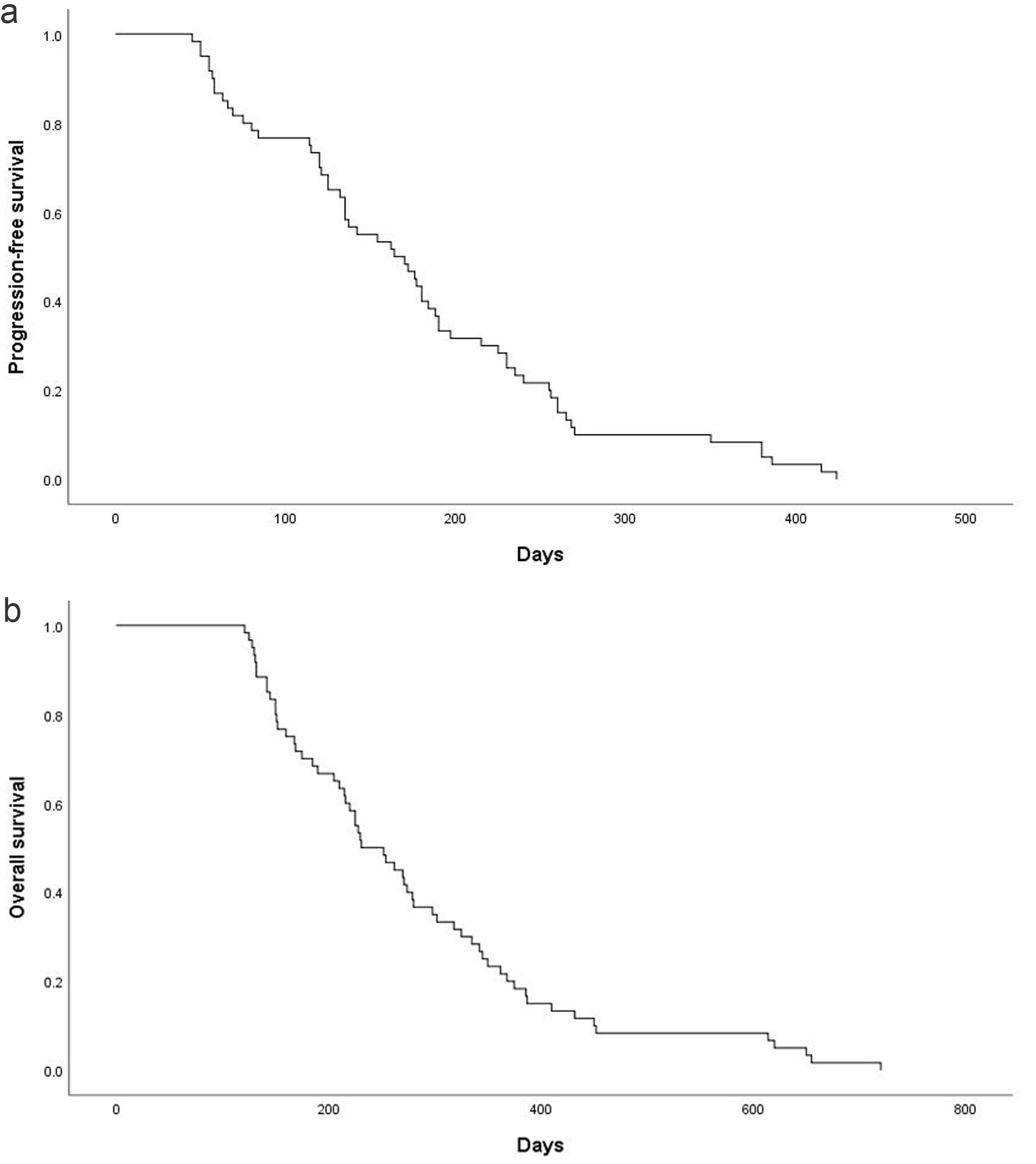
Figure 1. Kaplan-Meier estimates of PFS and OS. (a) PFS for the overall population and the median PFS was 175.8 ± 12.6 days (95% CI: 151.1 - 200.5). (b) OS for the overall population and the median OS was 280.4 ± 18.8 days (95% CI: 243.5 - 317.2). CI: confidence interval; OS: overall survival; PFS: progression-free survival.
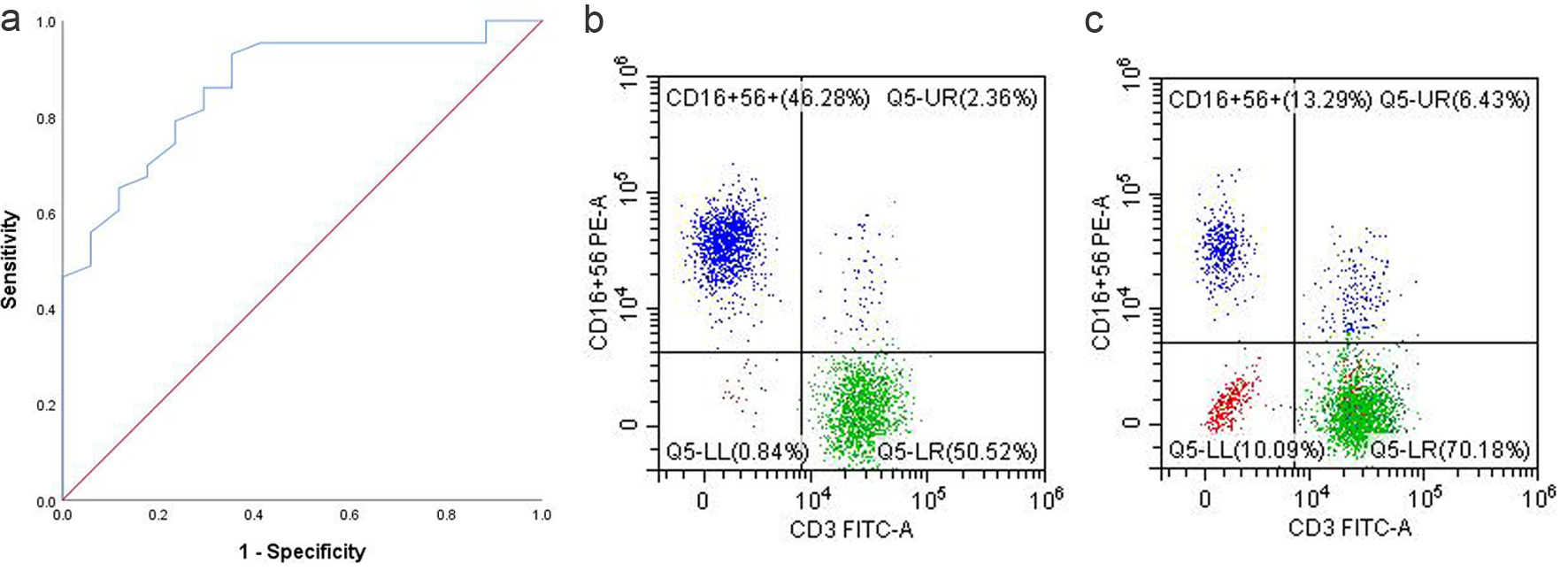
Figure 2. (a) ROC curve of NK cell proportion. (b) NK cell proportion in the PB of responders. (c) NK cell proportion in the PB of non-responders. NK: natural killer; PB: peripheral blood; ROC: receiver operating characteristic.
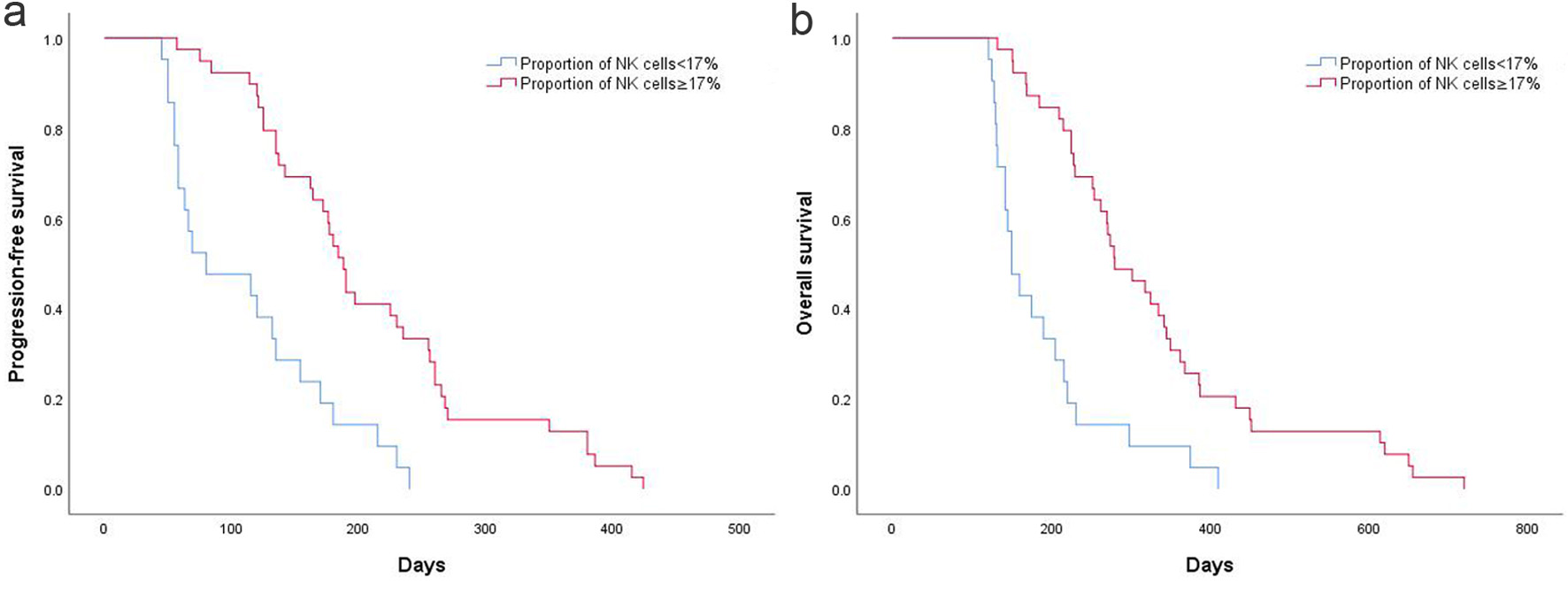
Figure 3. (a) High and low NK cell proportion on PFS in AGC patients. (b) High and low NK cell proportion on OS in AGC patients. AGC: advanced gastric cancer; NK: natural killer; OS: overall survival; PFS: progression-free survival.
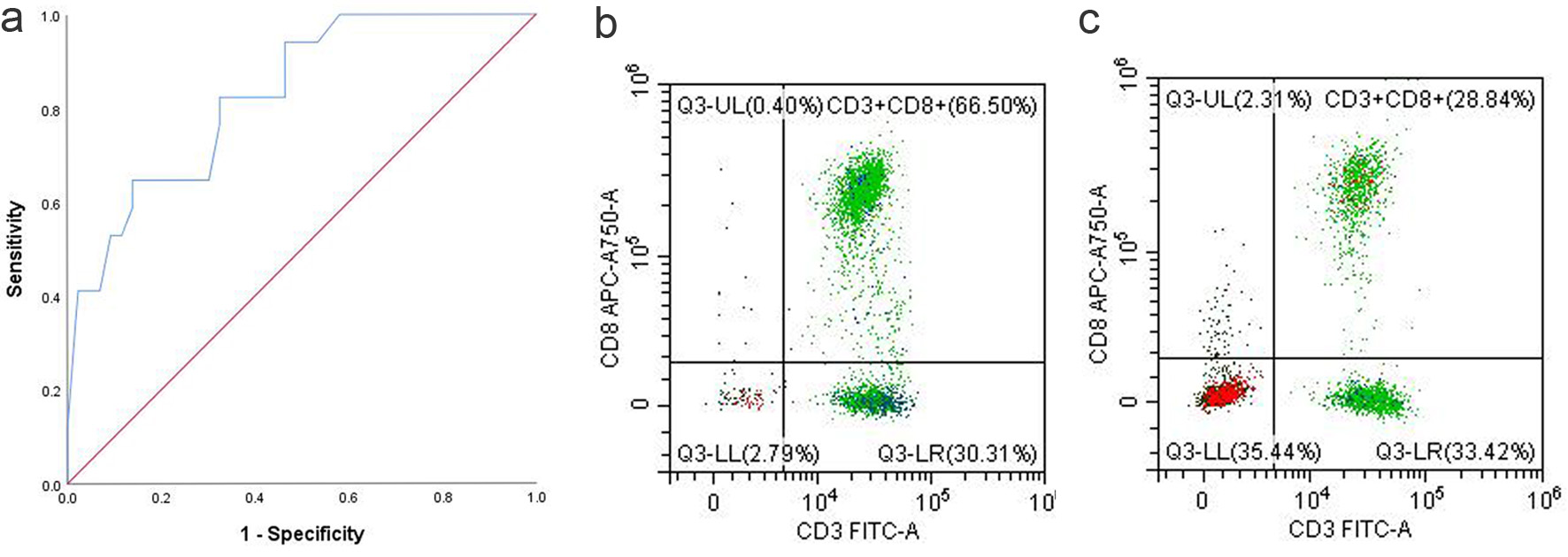
Figure 4. (a) ROC curve of CD8+ T-cell proportion. (b) CD8+ T proportion in the PB of responders. (c) CD8+ T proportion in the PB of non-responders. PB: peripheral blood; ROC: receiver operating characteristic.
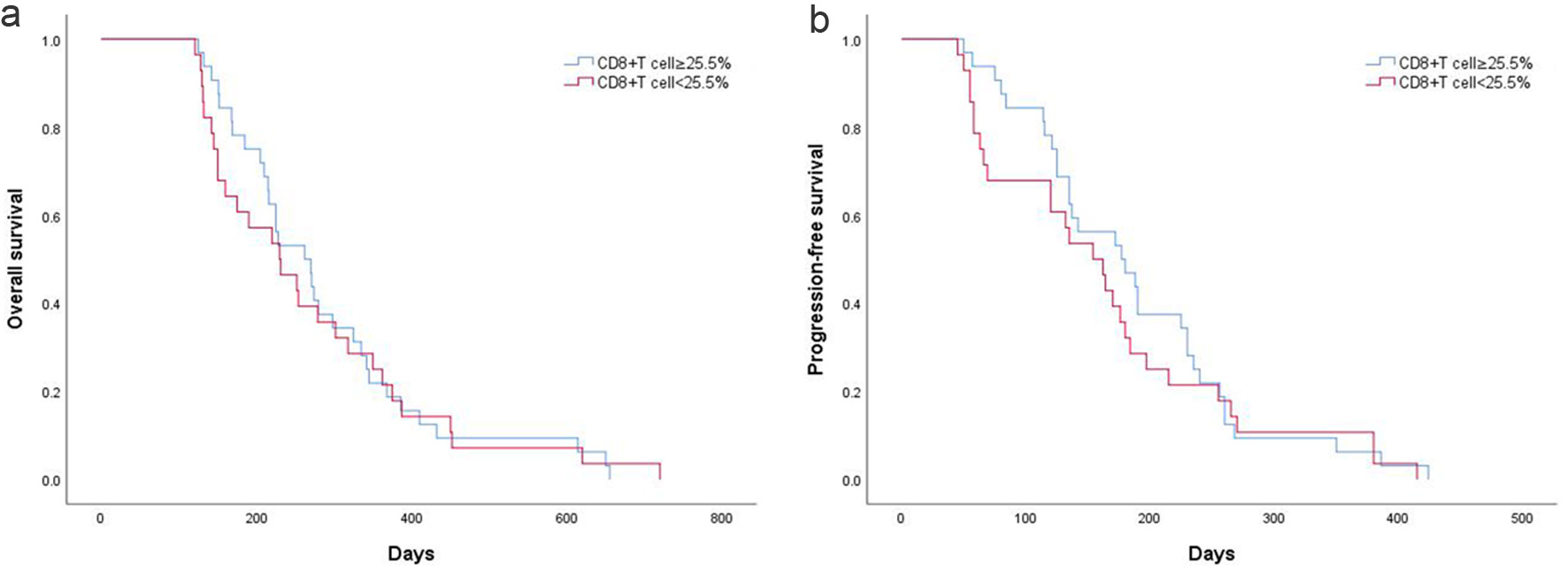
Figure 5. (a) PFS according to CD8+ T-cell proportion in AGC patients. (b) OS according to CD8+ T-cell proportion in AGC patients. AGC: advanced gastric cancer; OS: overall survival; PFS: progression-free survival.
Tables
Table 1. Characteristics of 60 AGC Patients
| Characteristics | n | % |
|---|
| AGC: advanced gastric cancer; ECOG PS: Eastern Cooperative Oncology Group performance status; SOX: S-1 and oxaliplatin. |
| Gender | | |
| Male | 36 | 60.0 |
| Female | 24 | 40.0 |
| Median age | 64.6 ± 8.3 years (range: 30 - 82) | |
| Age (years) | | |
| ≤ 65 | 32 | 53.3 |
| > 65 | 28 | 46.7 |
| ECOG PS | | |
| 0 | 45 | 75.0 |
| 1 | 10 | 16.7 |
| 2 | 5 | 8.3 |
| Primary lesion | | |
| Gastric | 43 | 71.7 |
| Gastroesophageal junction | 17 | 28.3 |
| Histology, n (%) | | |
| Intestinal type | 25 | 41.7 |
| Diffuse type | 9 | 15.0 |
| Mixed | 14 | 23.3 |
| Unknown | 12 | 20.0 |
| Metastatic sites, n | | |
| ≤ 2 | 32 | 53.3 |
| > 2 | 28 | 46.7 |
| Surgery of tumor | | |
| No | 22 | 36.7 |
| Yes | 38 | 63.3 |
| Initial dosage of apatinib (mg) | | |
| 500 | 24 | 40 |
| 250 | 36 | 60 |
| first-line chemotherapy regimen | | |
| SOX | 40 | 66.7 |
| Docetaxel plus S-1 | 9 | 15 |
| Fluoropyrimidine-oxaliplatin combination | 11 | 18.3 |
Table 2. Incidence of AEs During the Treatment
| AEs | Grade 1 or 2 (n) | Grade 3 or 4 (n) | Rate (%) |
|---|
| AEs: adverse events. |
| Hematologic | | | |
| Leukopenia | 27 | 3 | 50.0 |
| Anemia | 6 | 0 | 10.0 |
| Thrombocytopenia | 11 | 0 | 18.3 |
| Nonhematologic | | | |
| Fatigue | 27 | 4 | 50.7 |
| Hypertension | 27 | 2 | 48.3 |
| Proteinuria | 7 | 1 | 13.3 |
| Hand-foot syndrome | 13 | 1 | 23.3 |
| Diarrhea | 6 | 0 | 10.0 |




