| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 6, December 2025, pages 609-620
Efficacy of Tegafur-Uracil Maintenance Therapy in Non-Metastatic Head and Neck Squamous Cell Carcinoma: A Meta-Analysis With Trial Sequential Analysis
Hsu-Lin Leea, b, c, Cho-Hao Leeb, c, Ming-Shen Daib, c, Yueng-Hsiang Chud, Jih-Chin Leed, Po-Huang Chenb, c, e, Jia-Hong Chenb, c, e
aDivision of Hematology and Oncology, Department of Internal Medicine, Taichung Armed Forces General Hospital, Taichung, Taiwan, Republic of China
bDivision of Hematology and Oncology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical University, Taipei, Taiwan, Republic of China
cDepartment of Oncology, Tri-Service General Hospital, National Defense Medical University, Taipei, Taiwan, Republic of China
dDepartment of Otolaryngology-Head and Neck Surgery, Tri-Service General Hospital, National Defense Medical University, Taipei, Taiwan, Republic of China
eCorresponding Authors: Po-Huang Chen and Jia-Hong Chen, Division of Hematology and Oncology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical University, Taipei, Taiwan, Republic of China
Manuscript submitted July 8, 2025, accepted September 15, 2025, published online October 31, 2025
Short title: UFT Maintenance Therapy in Non-Metastatic HNSCC
doi: https://doi.org/10.14740/wjon2639
| Abstract | ▴Top |
Background: Patients with non-metastatic head and neck squamous cell carcinoma (HNSCC) face high risks of recurrence after curative-intent treatment. Maintenance therapy aims to prolong disease control during this vulnerable period. Tegafur-uracil (UFT), an oral fluoropyrimidine with a favorable toxicity profile, has demonstrated efficacy in other solid tumors. This study systematically evaluated the survival benefits of UFT as a maintenance therapy in HNSCC.
Methods: A systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, with the protocol registered on the Open Science Framework. PubMed, Embase, and CENTRAL were searched through May 2025. Eligible studies included adult patients with non-metastatic HNSCC who received UFT after curative treatment, with comparison to observation or standard care. Hazard ratios (HRs) for progression-free survival (PFS) and overall survival (OS) were pooled using random-effects models. Trial sequential analysis (TSA) was performed to assess the conclusiveness of the findings.
Results: Six observational studies including 1,373 patients were analyzed. UFT maintenance therapy was associated with improved PFS (HR: 0.68; 95% confidence interval (CI): 0.56 - 0.81; P < 0.001) and OS (HR: 0.54; 95% CI: 0.42 - 0.71; P < 0.001) compared with observation. TSA suggested that the available evidence may be sufficient to support a potential benefit for PFS, while for OS, further studies are still needed to strengthen the conclusions. Sensitivity analyses showed broadly consistent results; however, most included studies carried a moderate to serious risk of bias due to their non-randomized designs, which warrants cautious interpretation.
Conclusions: UFT maintenance therapy may be associated with improved survival outcomes in patients with non-metastatic HNSCC. Its oral formulation, relatively low toxicity, and potential cost-effectiveness suggest that it could represent a feasible treatment option. However, these observations currently indicate only a possible trend and should not be regarded as definitive conclusions. Further prospective randomized controlled trials are required to validate the efficacy of UFT maintenance therapy and to establish standardized protocols for patient selection and dosing.
Keywords: Head and neck; Squamous cell carcinoma; Tegafur-uracil; Maintenance therapy; Meta-analysis
| Introduction | ▴Top |
Head and neck squamous cell carcinoma (HNSCC) represents the sixth most common malignancy worldwide, with approximately 890,000 new cases diagnosed annually [1]. Despite significant advances in multimodal treatment approaches, including surgical resection, radiotherapy, and chemotherapy, the prognosis for patients with locally advanced disease remains challenging, with 5-year overall survival (OS) rates ranging from 40% to 65% depending on disease stage and anatomical site [2, 3].
The standard treatment paradigm for non-metastatic HNSCC typically involves either surgical resection followed by adjuvant chemoradiotherapy or definitive concurrent chemoradiotherapy (CCRT) for unresectable tumors [4, 5]. While these intensive curative-intent treatments can achieve complete remission in a substantial proportion of patients, the post-treatment period presents a critical therapeutic dilemma. Following completion of primary treatment, patients with no evidence of disease enter a surveillance phase consisting primarily of clinical observation with regular imaging and physical examinations [6]. However, despite achieving initial disease control, a significant proportion of patients eventually experience disease recurrence, with locoregional failure rates of 50-60% and distant metastasis rates of 10-25% within the first 2 years [7].
The concept of maintenance therapy - administering less intensive treatment following completion of definitive therapy - has shown benefits in various malignancies by prolonging disease-free intervals while maintaining acceptable quality of life [8, 9]. The challenge lies in identifying appropriate maintenance strategies that can prolong disease-free survival without compromising quality of life or imposing prohibitive toxicity. Conventional intensive chemotherapy regimens used during primary treatment are generally not suitable for maintenance therapy due to their significant toxicity profile, which would be poorly tolerated by patients in remission [10]. Similarly, while targeted therapies and immunotherapeutic agents have shown promise in various oncological settings, their widespread implementation as maintenance therapy is often limited by substantial financial burden and accessibility issues, particularly in resource-limited healthcare systems.
Tegafur-uracil (UFT), an oral fluoropyrimidine consisting of tegafur and uracil in a 1:4 molar ratio, represents an attractive option for maintenance therapy in HNSCC. Tegafur serves as a prodrug of 5-fluorouracil (5-FU), while uracil acts as a competitive inhibitor of dihydropyrimidine dehydrogenase, the rate-limiting enzyme in 5-FU catabolism, thereby enhancing the bioavailability and prolonging the half-life of the active metabolite [11]. This formulation allows for oral administration with sustained 5-FU levels while maintaining a favorable toxicity profile compared to intravenous 5-FU-based regimens [12].
The potential advantages of UFT as maintenance therapy include its oral bioavailability, manageable side effect profile, established efficacy in other malignancies, and relatively affordable cost. Previous studies have demonstrated the efficacy of UFT in various solid tumors, including colorectal [13-15], and lung cancers [16], where it has been successfully employed as both adjuvant and maintenance therapy. In the context of HNSCC, UFT offers the theoretical benefit of sustained antineoplastic activity during the high-risk period following primary treatment completion, potentially preventing micrometastatic disease progression while maintaining patient quality of life and treatment compliance [17, 18].
However, the evidence for UFT maintenance therapy in HNSCC has been derived primarily from individual institutional experiences and retrospective analyses, with limited systematic evaluation of its efficacy and safety profile. The heterogeneity in study designs, patient populations, treatment protocols, and outcome measures has made it challenging to draw definitive conclusions about the role of UFT maintenance therapy in HNSCC management.
Given the significant clinical need for effective maintenance strategies in HNSCC and the potential benefits of UFT therapy, a comprehensive systematic review and meta-analysis is warranted to synthesize the available evidence and provide clinicians with evidence-based guidance for treatment decision-making. Therefore, we conducted this systematic review and meta-analysis with trial sequential analysis (TSA) to evaluate the efficacy of UFT maintenance therapy in improving progression-free survival (PFS) and OS outcomes in patients with non-metastatic HNSCC who have completed curative-intent treatment.
| Materials and Methods | ▴Top |
Study design and protocol registration
This systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (Supplementary Material 1, wjon.elmerpub.com). The research protocol was prospectively registered with the Open Science Framework (OSF) to ensure methodological transparency and minimize reporting bias. This study was approved by the Ethics Review Boards of Tri-Service General Hospital (No. B202405200; approval date: December 18, 2024).
Ethical compliance with human study
All procedures involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee, as well as the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Literature search strategy
A comprehensive search was performed across three major electronic databases: PubMed (MEDLINE), Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL). The search timeframe extended from database inception through May 1, 2025, with no language restrictions applied initially, though only English-language publications were ultimately included for analysis.
The search strategy employed a combination of Medical Subject Headings (MeSH) terms and free-text keywords related to three core concepts: HNSCC, UFT therapy, and maintenance treatment approaches. Boolean operators (AND, OR) were used to combine search terms appropriately. The detailed search algorithms for each database are provided (Supplementary Material 2, wjon.elmerpub.com).
Additional studies were identified through manual review of reference lists from included articles, relevant systematic reviews, and conference abstracts from major oncology meetings. Forward citation searching was also performed for key studies to identify any additional relevant publications.
Study inclusion and exclusion criteria
Studies were considered eligible for inclusion if they met the following criteria: 1) enrolled adult patients (≥ 18 years) with histologically confirmed non-metastatic HNSCC who had completed curative-intent treatment; 2) investigated UFT as maintenance or adjuvant therapy in the post-treatment setting; 3) included a comparison group receiving either observation, placebo, or alternative maintenance therapy; 4) reported time-to-event outcomes including PFS and/or OS with sufficient data to calculate hazard ratios (HRs); and 5) employed either randomized controlled trial or observational cohort study designs.
Exclusion criteria included: 1) studies focusing exclusively on recurrent or metastatic disease; 2) single-arm studies without appropriate control groups; 3) case reports, case series, review articles, or editorials; 4) studies that did not report relevant survival endpoints; 5) conference abstracts without full-text publication; 6) duplicate publications reporting on identical patient populations; and 7) studies focusing solely on quality of life or toxicity outcomes without survival data.
Study selection process
Two investigators independently screened all identified records using a two-stage process following PRISMA guidelines. Initially, titles and abstracts were reviewed for potential relevance using predefined screening criteria. Subsequently, full-text articles of potentially eligible studies were obtained and assessed against the predetermined inclusion and exclusion criteria. Any disagreements between reviewers were resolved through discussion, with consultation of a third senior investigator when consensus could not be reached. The study selection process is illustrated in the PRISMA flow diagram (Fig. 1).
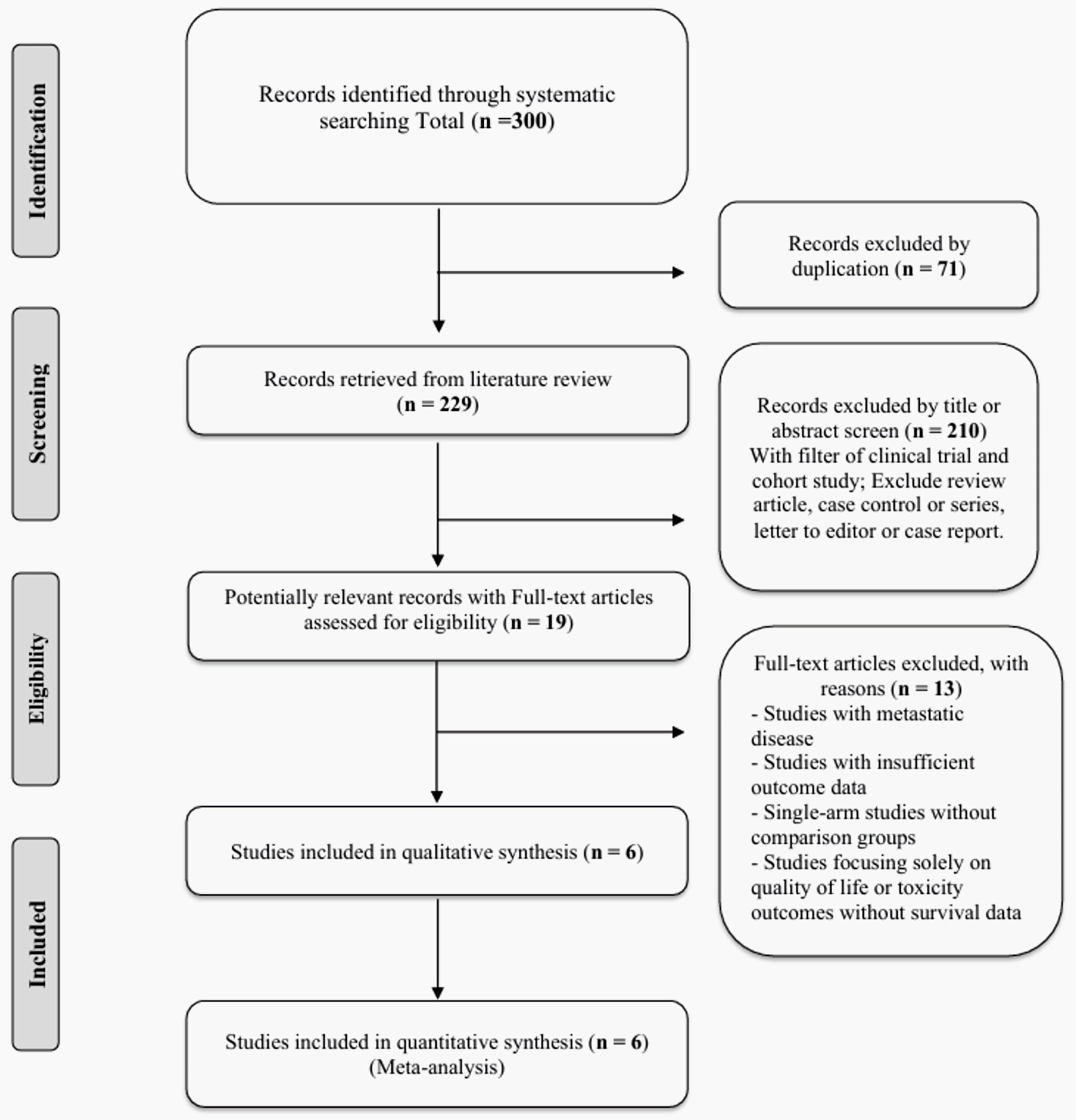 Click for large image | Figure 1. PRISMA flow diagram for study selection. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
Data extraction methodology
Standardized data extraction forms were developed and pilot-tested before implementation. Two reviewers independently extracted relevant information from each included study, including: study design and setting; patient demographics and baseline characteristics (age, gender, Eastern Cooperative Oncology Group (ECOG) performance status); tumor staging and histological details (TNM classification, primary tumor site); prior treatment modalities (surgery, radiotherapy, chemotherapy regimens); UFT dosing regimens and treatment duration; control group interventions; outcome definitions and measurement methods; follow-up duration; and statistical analysis approaches.
When necessary, authors of included studies were contacted to obtain additional data or clarification of methodology. For studies with multiple publications, the most recent or comprehensive report was used as the primary source, with earlier publications consulted for supplementary information.
Quality assessment framework
The methodological quality of included studies was evaluated using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool, as all included studies were observational cohort studies. The ROBINS-I assessment evaluated seven domains: bias due to confounding, bias in selection of participants, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes, and bias in selection of reported results.
Quality assessment was performed independently by two reviewers, with disagreements resolved through consensus discussion. Overall quality ratings were assigned as low, moderate, serious, or critical risk of bias for each study. Detailed risk of bias assessments for all included studies is provided (Supplementary Material 3, wjon.elmerpub.com).
Statistical analysis approach
For the meta-analysis methodology, HRs with 95% confidence intervals (CIs) served as the primary effect measure for time-to-event outcomes (PFS and OS). Meta-analysis was conducted using both fixed-effect and random-effects models, with random-effects results presented as the primary analysis due to anticipated clinical and methodological heterogeneity among studies.
The DerSimonian-Laird method was employed for random-effects meta-analysis, with between-study variance (tau2) estimated using the restricted maximum likelihood approach. Statistical significance was assessed at the 0.05 level.
For heterogeneity assessment, statistical heterogeneity was quantified using the I2 statistic, with values of 25%, 50%, and 75% representing low, moderate, and high heterogeneity, respectively. The Chi-square test (Cochran’s Q) was used to assess the statistical significance of heterogeneity, with P < 0.10 considered indicative of substantial heterogeneity given the low power of this test.
For sensitivity analyses, the robustness of findings was evaluated through sensitivity analyses using the leave-one-out approach, where each study was sequentially excluded to assess its influence on the overall effect estimate. These analyses are presented here (Supplementary Material 4, wjon.elmerpub.com) to demonstrate the stability of the meta-analysis results.
For publication bias assessment, assessment of publication bias using funnel plot visual inspection and Egger’s regression test was not performed due to the limited number of included studies (< 10 studies). According to methodological guidelines, these tests require at least 10 studies for reliable interpretation, as the test power is insufficient to distinguish chance from real asymmetry with fewer studies. This limitation is discussed (Supplementary Material 5, wjon.elmerpub.com).
For TSA, to address concerns about random errors due to sparse data and repeated significance testing, TSA was performed using TSA software version 0.9.5.10 Beta (Copenhagen Trial Unit, Denmark). This analysis aimed to determine whether the cumulative evidence was sufficient to support firm conclusions or whether additional studies were needed. TSA was conducted using a two-sided alpha level of 5%, power of 80%, and a clinically relevant relative risk reduction of 20% based on expert clinical judgment and literature review. The required information size was calculated based on the assumed intervention effect, control group event rate, and desired statistical power.
For software and statistical packages, all statistical analyses were performed using R statistical software version 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria) with the “meta” and “metafor” packages for meta-analysis. Forest plots were generated using standard R graphics packages.
A two-sided P value < 0.05 was considered statistically significant for all analyses, except for heterogeneity testing where P < 0.10 was used as the threshold.
| Results | ▴Top |
Study selection and characteristics
The systematic literature search identified 300 records from the three electronic databases (PubMed, Embase, and Cochrane Central). After removing 71 duplicate records, 229 unique citations remained for title and abstract screening. Following the application of inclusion and exclusion criteria, 210 records were excluded during the initial screening phase, primarily due to study design limitations (review articles, case series, single-arm studies) or focus on metastatic disease. Nineteen full-text articles were assessed for eligibility, of which 13 were subsequently excluded due to metastatic disease, insufficient outcome data, lack of comparison groups, or focus solely on quality of life/toxicity outcomes without survival data. Ultimately, six studies met all inclusion criteria and were included in both qualitative synthesis and quantitative meta-analysis (Fig. 1).
It should be noted that the search did not yield eligible studies that focused on UFT maintenance therapy following a primary treatment strategy of induction chemotherapy; therefore, the subsequent analysis is confined to patients who completed definitive or adjuvant (postoperative) treatment regimens.
Study characteristics and patient demographics
The six included studies were all observational cohort studies conducted between 2018 and 2025, encompassing a total of 1,373 patients with non-metastatic HNSCC [9, 19-23]. Sample sizes ranged from 93 to 424 patients per study. All studies investigated UFT maintenance therapy following curative-intent treatment compared to observation.
The studies included patients with oral cavity, oropharynx, hypopharynx, and laryngeal cancers. The majority of patients had locally advanced diseases (stage III-IV). Prior treatments included surgical resection followed by adjuvant chemoradiotherapy in most studies, with one study focusing on definitive chemoradiotherapy without surgery (Table 1) [9, 19-23]. Patient demographics showed male predominance (88-98%) and mean age ranging from 49 to 57 years. The UFT and control groups were generally well-balanced for baseline characteristics (Table 2) [9, 19-23].
 Click to view | Table 1. Characteristics of Studies Evaluating UFT Maintenance Therapy in Advanced Head and Neck Cancers |
 Click to view | Table 2. Patient Demographics and Disease Characteristics in UFT Maintenance Therapy Studies |
UFT dosing regimens varied across studies, with most employing 300 - 400 mg daily divided into two doses, with treatment duration typically planned for 1 year. Treatment compliance data were not consistently reported across all studies.
Risk of bias assessment
All included studies were assessed using the ROBINS-I tool for non-randomized studies. Overall risk of bias was rated as serious in five studies and moderate in one study. The primary sources of bias were confounding (due to non-randomized treatment allocation) and participant selection. Most studies attempted to control for confounding through multivariable analysis, adjusting for factors such as age, tumor stage, nodal status, and performance status. However, residual confounding remained a concern due to the observational nature of all included studies (Supplementary Material 3, wjon.elmerpub.com).
Meta-analysis results
PFS
Five studies with 1,280 patients provided data for PFS analysis. The meta-analysis demonstrated a statistically significant improvement in PFS with UFT maintenance therapy compared to observation (HR: 0.68, 95% CI: 0.56 - 0.81, P < 0.001). Heterogeneity was moderate (I2 = 32.7%, P = 0.20), suggesting reasonable consistency across studies despite methodological differences (Fig. 2).
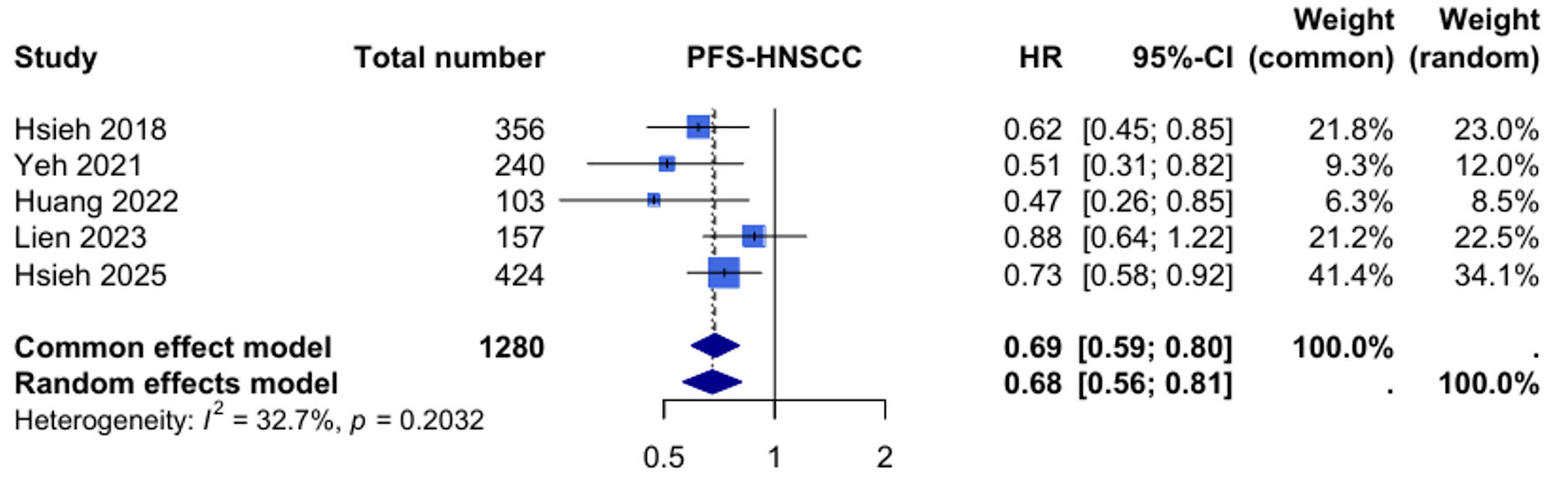 Click for large image | Figure 2. Forest plot for progression-free survival (PFS) in head and neck squamous cell carcinoma (HNSCC). HR: hazard ratio; CI: confidence interval. |
TSA for PFS was conducted with an assumed relative risk reduction of 20%, alpha of 5%, and power of 80%. The required information size was calculated to be 1,413 patients. With the current evidence including 1,280 patients (90.6% of required information size), the Z-curve crossed both the conventional boundary for benefit and the trial sequential monitoring boundary, suggesting that sufficient evidence exists to conclude a beneficial effect of UFT maintenance therapy on PFS (Fig. 3).
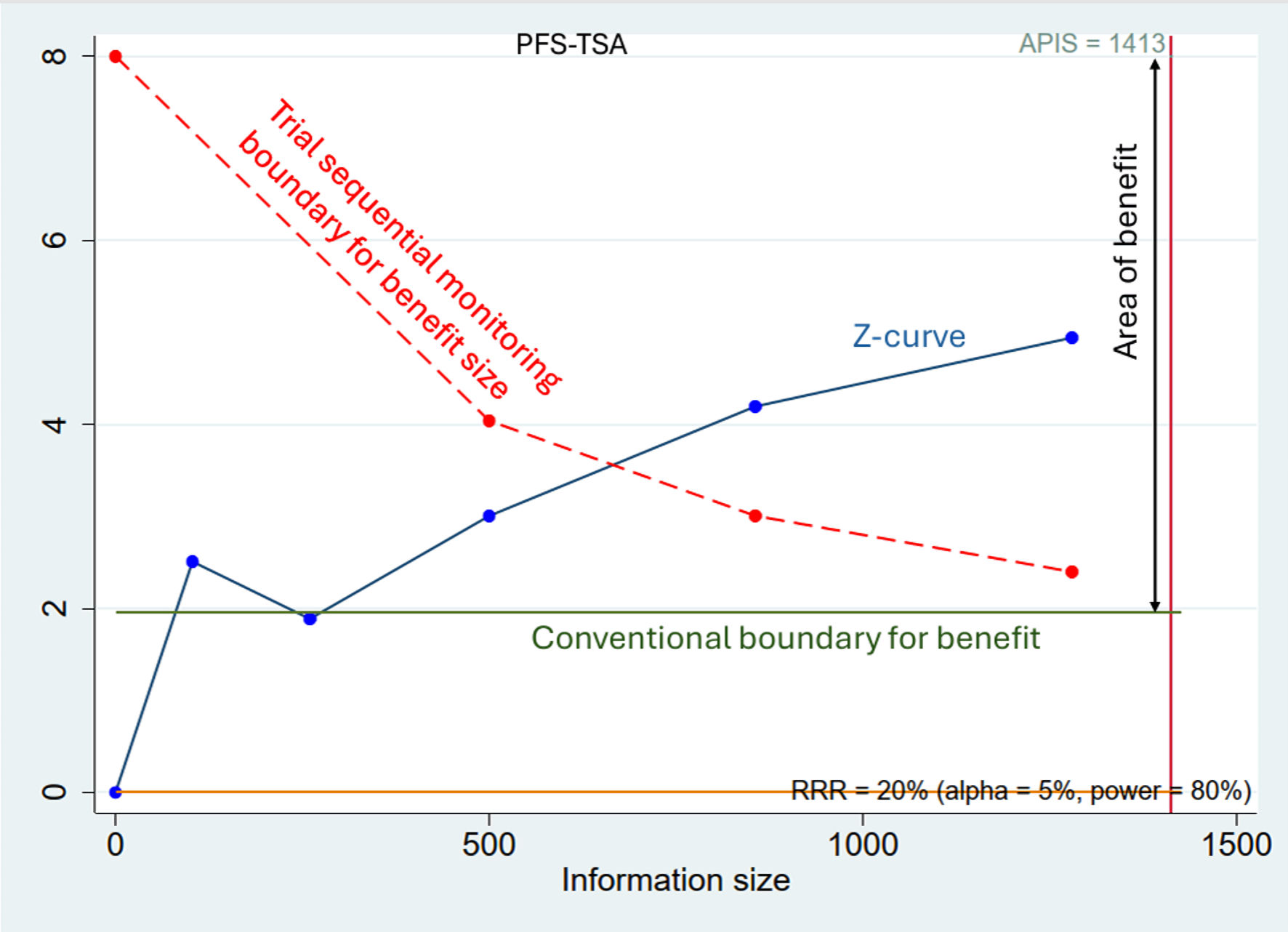 Click for large image | Figure 3. Trial sequential analysis for progression-free survival (PFS-TSA). The blue line represents the cumulative Z-curve, which crosses both the conventional boundary for benefit (inner green line, Z = 1.96) and the trial sequential monitoring boundary for benefit (outer dashed red line). The cumulative sample size (1,280 patients) has reached 90.6% of the required information size (APIS = 1,413 patients, vertical red line), suggesting that the evidence for a benefit in PFS is sufficient and conclusive. |
OS
Six studies with 1,373 patients contributed to the OS analysis. UFT maintenance therapy was associated with a significant improvement in OS compared to observation (HR: 0.54, 95% CI: 0.42 - 0.71, P < 0.001). Statistical heterogeneity was moderate to high (I2 = 55.7%, P = 0.046), indicating some variability in treatment effects across studies (Fig. 4).
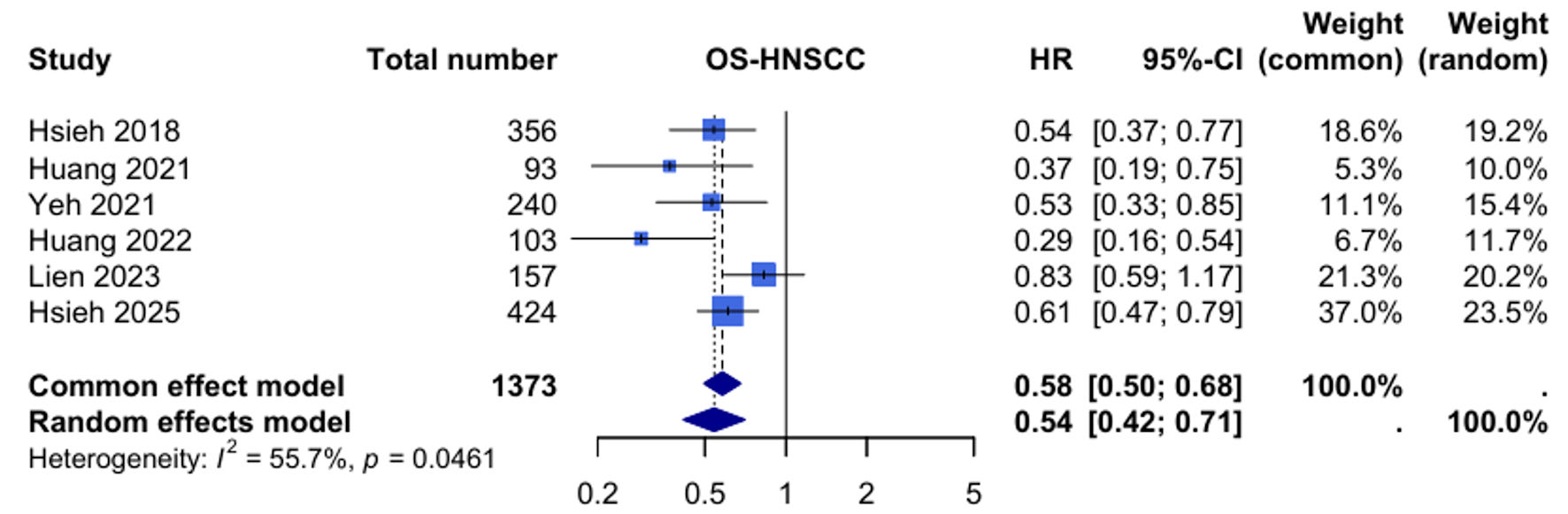 Click for large image | Figure 4. Forest plot for overall survival (OS) in head and neck squamous cell carcinoma (HNSCC). HR: hazard ratio; CI: confidence interval. |
For OS-TSA, the required information size was calculated to be 1,816 patients. The current meta-analysis included 1,373 patients (75.6% of required information size). While the Z-curve crossed the conventional boundary for benefit, the trial sequential monitoring boundary was not reached. Consequently, the evidence to date is inconclusive, and any potential survival benefit should be regarded as provisional until confirmed by larger, high-quality trials (Fig. 5).
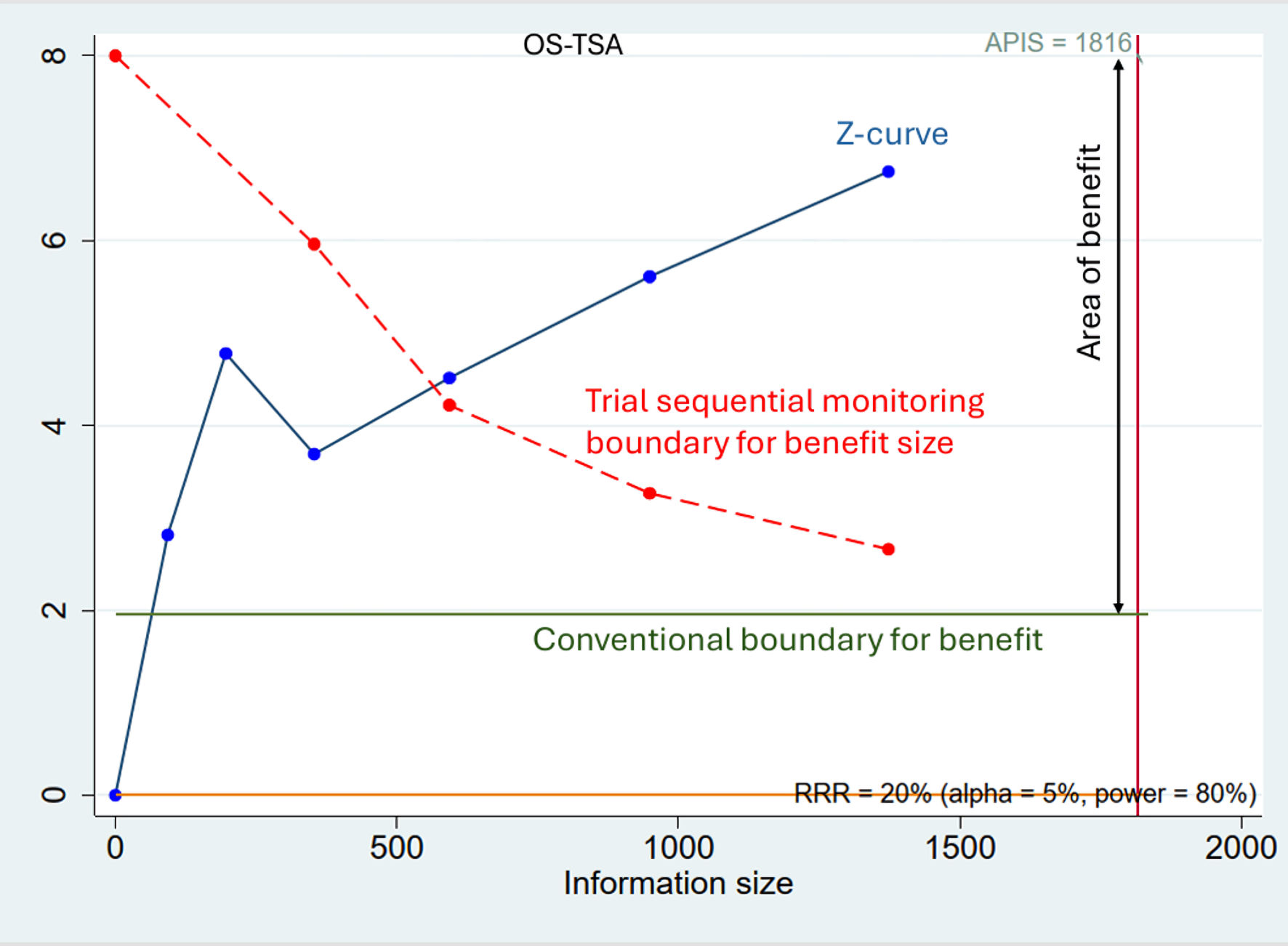 Click for large image | Figure 5. Trial sequential analysis for overall survival (OS-TSA). The blue line represents the cumulative Z-curve. Although the Z-curve has crossed the conventional boundary for benefit (inner green line, Z = 1.96), it has not yet crossed the more stringent trial sequential monitoring boundary (outer dashed red line). The cumulative sample size (1,373 patients) has only reached 75.6% of the required information size (APIS = 1,816 patients, vertical red line). This indicates that while there is a trend towards benefit, the evidence is not yet conclusive, and further studies are needed. |
Sensitivity analysis
Sensitivity analyses using the leave-one-out approach demonstrated the robustness of the meta-analysis findings for both outcomes. For PFS, sequential exclusion of individual studies resulted in HRs ranging from 0.65 to 0.71, with all CIs remaining statistically significant. For OS, the HRs ranged from 0.53 to 0.61, again maintaining statistical significance regardless of which study was excluded. These analyses indicate that no single study disproportionately influenced the overall results (Supplementary Material 4, wjon.elmerpub.com).
Publication bias assessment
Publication bias assessment via funnel plots and Egger’s test was not performed due to the limited number of included studies (< 10 studies). According to methodological guidelines, tests for funnel plot asymmetry require at least 10 studies for reliable interpretation. Therefore, while publication bias cannot be ruled out, formal statistical assessment was deemed inappropriate given these limitations (Supplementary Material 5, wjon.elmerpub.com).
| Discussion | ▴Top |
This systematic review and meta-analysis provide, to our knowledge, the first comprehensive evaluation of UFT maintenance therapy in non-metastatic HNSCC. Based on six observational studies including 1,373 patients, our findings suggest that UFT maintenance therapy may be associated with improvements in both PFS and OS compared with observation alone following curative-intent treatment. TSA indicated that the available evidence appears adequate to suggest a potential benefit for PFS, whereas the findings for OS remain less certain and require confirmation through additional research. Overall, these results should be interpreted with caution, and further high-quality studies are necessary before UFT maintenance therapy can be considered for routine incorporation into standard care pathways for HNSCC.
Therapeutic benefits
Our meta-analysis lends preliminary support to the potential role of metronomic chemotherapy as implemented through UFT maintenance therapy. This approach, which involves continuous administration of chemotherapeutic agents at low, minimally toxic doses, has been hypothesized to inhibit tumor angiogenesis, modulate immune responses, and reduce the likelihood of tumor regrowth [24]. The sustained drug exposure achieved with UFT may contribute to targeting minimal residual disease and delaying the establishment of micrometastatic foci during the post-treatment period [25].
While the observed trends appear consistent across different primary tumor sites and treatment modalities, these findings should be interpreted with caution. The field cancerization phenomenon, characterized by multiple genetic alterations in the head and neck mucosa due to chronic exposure to carcinogens such as tobacco and alcohol, may predispose to recurrence [1]. UFT maintenance therapy could potentially help counteract these oncogenic processes through its sustained antineoplastic activity, although further high-quality studies are needed to confirm these effects.
It is crucial to interpret our findings in light of the observational design of all included studies, which carry a moderate to serious risk of bias (Supplementary Material 3, wjon.elmerpub.com). As treatment was allocated by physician discretion, selection bias favoring patients with better prognoses for UFT maintenance is a significant concern. Despite multivariable adjustments in the original studies, residual confounding likely influenced the outcomes. Therefore, the magnitude of the observed treatment effect (OS HR 0.54; PFS HR 0.68) is plausibly inflated by these inherent biases. While the consistency of the results is noteworthy, a definitive causal link between UFT and improved survival awaits validation from prospective randomized controlled trials.
Comparison with contemporary therapies and clinical implications
Compared to other oral fluoropyrimidines such as S-1, UFT demonstrates efficacy as a maintenance therapy in non-metastatic head and neck cancers that is not inferior, with distinct advantages. Similarly, the ACTS-HNC trial in HNSCC showed that 1 year of S-1 yielded a 3-year disease-free survival of 64.1%, comparable to UFT’s 60.0%, though S-1 achieved a higher 3-year OS (82.9% vs. 75.8%) [25]. As previously noted, UFT’s favorable toxicity profile and practical advantages - such as less hand-foot syndrome than capecitabine and reduced hematologic toxicity compared to S-1 - make it a suitable option for prolonged maintenance therapy.
The emergence of immunotherapy and targeted therapies has influenced current approaches to HNSCC treatment, yet these agents are primarily utilized in the recurrent or metastatic setting [26-28]. Comparison with EGFR-targeted therapy, such as cetuximab, UFT holds a more established role in maintenance therapy. The RTOG 0234 phase II trial demonstrated survival benefits when cetuximab was combined with postoperative chemoradiation in high-risk HNSCC, yet no phase III trials have confirmed its status as a standard maintenance therapy, and its toxicity (e.g., rash) limits widespread adoption [26]. By comparison, the findings of our study suggest that UFT may provide survival benefits with relatively lower toxicity, although these results remain to be validated in prospective randomized trials before its broader clinical utility can be established.
Regarding immune checkpoint inhibitors, their role varies by cancer type. In nasopharyngeal carcinoma (NPC), programmed cell death protein 1 (PD-1) inhibitors like camrelizumab (DIPPER trial) have shown significant benefits in the maintenance phase post-CCRT, improving 3-year event-free survival (86.9% vs. 77.4%) and reducing distant metastases, particularly in high-risk patients (e.g., those with elevated Epstein-Barr virus (EBV) DNA) [29]. This positions immunotherapy as a promising candidate for standard care in NPC, increasingly integrated into clinical practice. However, in HNSCC, evidence remains insufficient to support immunotherapy as a standard maintenance therapy [28]. The JAVELIN Head and Neck 100 trial with avelumab failed to improve PFS [30], and the KEYNOTE-412 trial has yet to demonstrate overwhelming benefits [28], suggesting further research is needed to clarify its role. Their application in HNSCC maintenance may also be limited by cost considerations and potential autoimmune toxicities in disease-free patients. Our findings suggest that UFT provides an immediately implementable maintenance strategy that bridges the therapeutic gap.
Implementation should consider patient selection criteria, optimal dosing strategies, and treatment duration. While the included studies employed varying protocols, most utilized UFT 300 - 400 mg daily divided into two doses for approximately 1 year. In addition, UFT with a reduced toxicity profile may be suitable for long-term maintenance therapy in the elderly and frail patients [31]. Therefore, the favorable toxicity profile allows for individualized dose modifications based on patient tolerability, ensuring treatment can be tailored to optimize both efficacy and quality of life.
Evidence on the safety profile of UFT from observational studies
In the studies included in our review, UFT appeared to be generally well tolerated. Huang et al reported no significant difference in adverse effects between UFT and non-UFT groups among patients with non-distant-metastatic stage IV oral cavity cancer, with oral UFT showing no grade 4 or 5 toxicities [19]. Huang et al observed that the most common adverse event was macrocytic anemia, while most toxicities were mild, infrequent, and typically manageable with dose reduction [20]. Yeh et al found that UFT-related toxicities were limited to grade 1-2 events with an overall prevalence of 17.7% [22]. Hsieh et al similarly indicated that adverse effects were generally mild, with no grade 4-5 toxicities, treatment-related deaths, severe hepatic dysfunction, or bone marrow suppression [9]. Overall, these findings suggest that UFT may be associated with a manageable safety profile, although the current evidence is limited and primarily based on observational studies.
Future research directions
Several important questions remain to be addressed through future research. The identification of predictive biomarkers could enable more precise patient selection for UFT maintenance therapy, potentially enhancing therapeutic efficacy while minimizing unnecessary treatment exposure. Combination strategies incorporating UFT with targeted therapies or immunotherapeutic agents warrant investigation, particularly given the complementary mechanisms of action these approaches might provide.
The optimal duration of UFT maintenance therapy requires further study, as does the potential for treatment breaks or dose de-escalation strategies in patients achieving sustained disease control. Future studies should incorporate robust assessments of quality of life and patient-reported outcomes to ensure that survival benefits translate into meaningful improvements in patient well-being, alongside systematic monitoring of treatment adherence.
Strengths and limitations
This meta-analysis has several notable strengths. We conducted a comprehensive search strategy across multiple databases and followed rigorous systematic review methodology with PRISMA guidelines. The inclusion of TSA provides additional statistical rigor and helps address concerns about random errors due to sparse data. Our sensitivity analyses demonstrated the robustness of findings, with no single study disproportionately influencing the overall results.
However, several limitations must be acknowledged. All included studies were observational cohort studies rather than randomized controlled trials, introducing potential confounding bias despite multivariable adjustments employed by individual studies. The non-randomized allocation to UFT maintenance therapy was largely based on physician discretion, which may have resulted in selection bias favoring patients with better prognosis or performance status for treatment allocation. Heterogeneity in UFT dosing regimens, treatment duration, and follow-up protocols across studies may have influenced the observed treatment effects.
The relatively small number of included studies precluded formal assessment of publication bias using statistical methods, though the consistent direction of effect across all studies reduces this concern. Additionally, the observational nature of included studies limits our ability to establish definitive causal relationships between UFT maintenance therapy and improved outcomes, though the consistency and magnitude of observed benefits provide compelling evidence for therapeutic efficacy.
All included studies were conducted in East Asia, primarily in Taiwan and Japan, which may limit the generalizability of the findings to other populations and clinical practice settings. UFT, originally developed by Japanese pharmaceutical companies, has been more widely used in Asia but is not routinely available or approved in many Western countries. These geographic and regulatory differences may partly account for the limited number of UFT-based trials outside East Asia. Therefore, the external validity of the current findings remains uncertain, and further studies in more diverse populations are needed to confirm their applicability.
Furthermore, crucial data for evaluating a maintenance therapy were not systematically reported across the included studies. Key metrics such as median follow-up durations, patient-reported quality of life outcomes, and detailed treatment adherence rates were either unavailable or inconsistently documented, limiting our ability to conduct a more granular analysis and to form a complete picture of the treatment burden.
Conclusions
This systematic review and meta-analysis suggest that UFT maintenance therapy may offer survival benefits for patients with non-metastatic HNSCC following curative-intent treatment. Pooled analyses indicate a potential improvement in PFS and OS compared to observation, although TSA shows that firm conclusions - particularly for OS - remain uncertain. While the consistent trends across studies, together with UFT’s favorable toxicity profile, oral administration route, and relative affordability, make it an attractive option, the current evidence is limited by the observational nature and methodological constraints of available studies. Therefore, UFT should be regarded as a promising but not yet established approach, and prospective randomized controlled trials are needed to confirm these findings, refine patient selection, and determine optimal dosing and treatment duration.
| Supplementary Material | ▴Top |
Suppl 1. PRISMA checklist for systematic review and meta-analysis.
Suppl 2. Search strategy and literature identification methods.
Suppl 3. Risk of bias assessment for included studies.
Suppl 4. Sensitivity analysis to assess robustness of meta-analysis results.
Suppl 5. Publication bias analysis: funnel plots and Egger’s test results.
Acknowledgments
None to declare.
Financial Disclosure
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
HLL and PHC collected the data and wrote the manuscript. CHL, MSD, YHC, JCL, and JHC revised the manuscript. All authors approved the final version of the manuscript.
Data Availability
The data presented in this study are available on request from the corresponding author.
| References | ▴Top |
- Barsouk A, Aluru JS, Rawla P, Saginala K, Barsouk A. Epidemiology, risk factors, and prevention of head and neck squamous cell carcinoma. Med Sci (Basel). 2023;11(2).
doi pubmed - Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92.
doi pubmed - Ionna F, Bossi P, Guida A, Alberti A, Muto P, Salzano G, Ottaiano A, et al. Recurrent/metastatic squamous cell carcinoma of the head and neck: a big and intriguing challenge which may be resolved by integrated treatments combining locoregional and systemic therapies. Cancers (Basel). 2021;13(10).
doi pubmed - Furness S, Glenny AM, Worthington HV, Pavitt S, Oliver R, Clarkson JE, Macluskey M, et al. Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. Cochrane Database Syst Rev. 2011;4:CD006386.
doi pubmed - Hutchinson MND, Mierzwa M, D'Silva NJ. Radiation resistance in head and neck squamous cell carcinoma: dire need for an appropriate sensitizer. Oncogene. 2020;39(18):3638-3649.
doi pubmed - Chen YP, Ismaila N, Chua MLK, Colevas AD, Haddad R, Huang SH, Wee JTS, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021;39(7):840-859.
doi pubmed - Denaro N, Merlano MC, Russi EG. Follow-up in head and neck cancer: do more does it mean do better? A systematic review and our proposal based on our experience. Clin Exp Otorhinolaryngol. 2016;9(4):287-297.
doi pubmed - Liu GY, Li WZ, Wang DS, Liang H, Lv X, Ye YF, Zhao C, et al. Capecitabine maintenance therapy after induction chemotherapy in newly diagnosed metastatic nasopharyngeal carcinoma: An open-label, randomized, controlled, phase trial. Journal of Clinical Oncology. 2021;39:6044.
doi - Hsieh MY, Chen G, Chang DC, Chien SY, Chen MK. The impact of metronomic adjuvant chemotherapy in patients with advanced oral cancer. Ann Surg Oncol. 2018;25(7):2091-2097.
doi pubmed - Dickstein DR, Lehrer EJ, Hsieh K, Hotca A, Jones BM, Powers A, Sharma S, et al. Management of older adults with locally advanced head and neck cancer. Cancers (Basel). 2022;14(11).
doi pubmed - Lee HL, Chen PH, Huang TC, Ye RH, Chu YH, Lee JC, Jhou HJ, et al. Tegafur-uracil maintenance therapy in non-metastatic head and neck cancer: an exploratory systematic review. Curr Oncol. 2025;32(5).
doi pubmed - Segura Huerta A, Diaz-Beveridge R, Perez-Fidalgo JA, Calderero Aragon V, Pastor Borgonon M, Aparicio Urtasun J, Montalar Salcedo J. Carboplatin and tegafur-uracil concomitant with standard radiotherapy in the management of locally advanced head and neck cancer. Clin Transl Oncol. 2005;7(1):23-28.
doi pubmed - Kato T, Ohashi Y, Nakazato H, Koike A, Saji S, Suzuki H, Takagi H, et al. Efficacy of oral UFT as adjuvant chemotherapy to curative resection of colorectal cancer: multicenter prospective randomized trial. Langenbecks Arch Surg. 2002;386(8):575-581.
doi pubmed - Sakamoto J, Hamada C, Yoshida S, Kodaira S, Yasutomi M, Kato T, Oba K, et al. An individual patient data meta-analysis of adjuvant therapy with uracil-tegafur (UFT) in patients with curatively resected rectal cancer. Br J Cancer. 2007;96(8):1170-1177.
doi pubmed - Kuo YH, Lai CH, Huang CY, Chen CJ, Huang YC, Huang WS, Chin CC. Monthly tegafur-uracil maintenance for increasing relapse-free survival in ypStage III rectal cancer patients after preoperative radiotherapy, radical resection, and 12 postoperative chemotherapy cycles: a retrospective study. BMC Cancer. 2019;19(1):815.
doi pubmed - Watanabe T, Tanahashi M, Suzuki E, Yoshii N, Kohama T, Iguchi K, Endo T. Efficacy of adjuvant tegafur-uracil (UFT) in early-stage non-small cell lung cancer with poor prognostic factors. Transl Lung Cancer Res. 2025;14(1):139-149.
doi pubmed - De Felice F, Musio D, Tombolini V. Head and neck cancer: metronomic chemotherapy. BMC Cancer. 2015;15:677.
doi pubmed - Su NW, Chen YJ. Metronomic Therapy in Oral Squamous Cell Carcinoma. J Clin Med. 2021;10(13).
doi pubmed - Huang WY, Ho CL, Chao TY, Lee JC, Chen JH. Oral tegafur-uracil as a metronomic therapy in stage IVa and IVb cancer of the oral cavity. Am J Otolaryngol. 2021;42(6):103156.
doi pubmed - Huang PW, Lin CY, Lee LY, Hsieh CH, Hsu CL, Liau CT, Fan KH, et al. Maintenance tegafur-plus-uracil after adjuvant concurrent chemoradiotherapy may improve outcome for resected oral cavity squamous cell carcinoma with extranodal extension. Front Oncol. 2022;12:866890.
doi pubmed - Lien CF, Hwang TZ, Lin TM, Liu KW, Lin BS, Wang CC, Yang CC, et al. Prognostic impact of cortactin in patients with hypopharyngeal cancer and its role for tegafur-uracil maintenance after adjuvant chemoradiotherapy. Am J Cancer Res. 2023;13(11):5504-5512.
pubmed - Yeh TJ, Chan LP, Tsai HT, Hsu CM, Cho SF, Pan MR, Liu YC, et al. The overall efficacy and outcomes of metronomic tegafur-uracil chemotherapy on locally advanced head and neck squamous cell carcinoma: a real-world cohort experience. Biology (Basel). 2021;10(2).
doi pubmed - Hsieh JC, Lien MY, Chang PH, Wang HM, Yeh KY, Ho CL, Hsieh CY, et al. UFUR maintenance therapy significantly improves survival in locally advanced head and neck squamous cell carcinoma following definitive chemoradiotherapy. Cancer Cell Int. 2025;25(1):187.
doi pubmed - Chen PH, Jhou HJ, Chung CH, Wu YY, Huang TC, Lee CH, Chien WC, et al. Benefit of uracil-tegafur used as a postoperative adjuvant chemotherapy for stage IIA colon cancer. Medicina (Kaunas). 2022;59(1).
doi pubmed - Tachibana M, Yasuda N, Yoshimatsu M, Nishimura H, Mizukoshi O. UFT for head and neck cancers: its tissue concentrations and effects on lymphocyte subpopulations. Cancer Chemother Pharmacol. 1987;19(1):65-68.
doi pubmed - Harari PM, Harris J, Kies MS, Myers JN, Jordan RC, Gillison ML, Foote RL, et al. Postoperative chemoradiotherapy and cetuximab for high-risk squamous cell carcinoma of the head and neck: Radiation Therapy Oncology Group RTOG-0234. J Clin Oncol. 2014;32(23):2486-2495.
doi pubmed - Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, Harrington K, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22(4):450-462.
doi pubmed - Machiels JP, Tao Y, Licitra L, Burtness B, Tahara M, Rischin D, Alves G, et al. Pembrolizumab plus concurrent chemoradiotherapy versus placebo plus concurrent chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck (KEYNOTE-412): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2024;25(5):572-587.
doi pubmed - Ma J, Sun Y, Liang YL, Liu X, Shen L, Hu W, Hu G, et al. Adjuvant PD-1 blockade with camrelizumab in high-risk locoregionally advanced nasopharyngeal carcinoma (DIPPER): A multicenter, open-label, phase 3, randomized controlled trial. J Clin Oncol. 2024;42:LBA6000.
doi - Yu Y, Lee NY. JAVELIN Head and Neck 100: a Phase III trial of avelumab and chemoradiation for locally advanced head and neck cancer. Future Oncol. 2019;15(7):687-694.
doi pubmed - Hsieh MC, Wang CC, Yang CC, Lien CF, Wang CC, Shih YC, Yeh SA, et al. Tegafur-uracil versus 5-fluorouracil in combination with cisplatin and cetuximab in elderly patients with recurrent or metastatic head and neck squamous cell carcinoma: a propensity score matching analysis. Biology (Basel). 2021;10(10).
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.