| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 000, Number 000, October 2025, pages 000-000
Measuring the Unseen Burden: Immunotherapy and Targeted Therapy Reduce Treatment-Related Time Toxicity in Incurable Malignancies
Yuhang Zhoua, b , Madeline Fitzpatricka, Sujata Ojhaa, Marisabel Hurtado-Castilloa, Williams Sessionsa, Kyaw Aunga, Om Pandeya, Boone Goodgamea
aDepartment of Hematology and Oncology, Dell Medical School, University of Texas at Austin, Austin, TX, USA
bCorresponding Author: Yuhang Zhou, Department of Hematology and Oncology, Dell Medical School, University of Texas at Austin, Austin, TX 78723, USA
Manuscript submitted July 6, 2025, accepted September 13, 2025, published online October 10, 2025
Short title: Time Toxicity in Incurable Cancer Care
doi: https://doi.org/10.14740/wjon2637
| Abstract | ▴Top |
Background: Treatment-related time toxicity (TrTT) poses a cumulative burden on quality of life. We aimed to analyze and quantify the impact of the TrTT on patients with incurable malignancies in our ambulatory oncology clinic.
Methods: We performed a retrospective observational analysis of the TrTT for individuals with incurable solid cancers at a regional safety-net oncology office that focuses on underserved communities. Time toxicity is calculated as the number of days a patient spent with any healthcare-related encounters during the initial 12-week period of the most recent line of therapy from our medical record system.
Results: Among the 150 patients included, the median TrTT was 13 days/12 weeks (interquartile range (IQR) 7 - 23). Fourteen percent of them experienced severe TrTT (> 1/3 of the time spent seeking treatment). We identified multiple risk factors for higher TrTT, including poor performance status, the need for cytotoxic chemotherapy, and gastrointestinal malignancies. Immunotherapy, targeted therapy, and hormone therapy were associated with significantly lower TrTT when compared with cytotoxic chemotherapy.
Conclusions: Disproportionally high time toxicity was seen in patients with frailty, had gastrointestinal malignancies, and were receiving cytotoxic chemotherapy. Our findings underscore the importance of including time-based metrics in shared decision-making in the palliative treatment setting.
Keywords: Time toxicity; Incurable cancer; Healthcare utilization; Immunotherapy; Targeted therapy
| Introduction | ▴Top |
The time spent seeking cancer care poses a substantial burden on patients’ quality of life, especially for those with incurable malignancies [1-3]. Compared with the general population, oncology patients typically utilize the healthcare more frequently. For example, individuals in the USA visit their primary care physician on average three times a year according to the Centers for Disease Control and Prevention (CDC) data, whereas some cancer patients receive weekly chemotherapy infusions with laboratory tests. Time toxicity describes the cumulative burden of time patients endure while pursuing medical care, including travel time, obtaining diagnostic scans and biopsies, office visits, time spent receiving infusion or radiation, hospitalizations, laboratory visits, follow-up appointments, and side effect management [3, 4]. Time toxicity exists for all aspects of healthcare, and was previously described as time cost, lost day, healthcare contact day, and opportunity cost [5-8]. Time toxicity can significantly affect patients’ lives beyond the clinical impact of cancer itself. Frequent appointments, long travel times, and extended hours spent in waiting rooms or undergoing tests often disrupt daily routines and reduce the amount of time patients can spend at home or engaging in meaningful activities. This loss of autonomy over one’s time can be emotionally distressing, particularly for patients with a limited life expectancy who may value quality time with loved ones over marginal clinical gains. Despite the tremendous progress made in cancer treatment, most of the guideline-recommended palliative treatment options convey short survival benefits of approximately 3 - 6 months when compared with supportive care [9]. While medication toxicities are meticulously reviewed with patients, the cumulative impact of time is rarely included in the discussion, partly due to the lack of standardized measurement of time toxicity, and the lack of data from clinical trials. Thus, there is a compelling need for the quantification of time toxicity to guide clinical practice and patient preference.
Prior studies have confirmed that individuals with cancer face a large burden of time dealing with the health care system. A Mexican study demonstrated that palliative systemic therapy consumed on average 1 in 5 days in patients older than 65 years with metastatic cancer during the first 6 months of treatment, with higher toxicity in the cohorts receiving radiotherapy or chemotherapy [1]. A Canadian study of 5,785 patients with stage IV non-small cell lung cancer estimated that 1 in 3 days were spent interacting with the health care system outside of home, with fewer days consumed when receiving targeted therapy [2]. Similar patterns were also found in advanced gastrointestinal cancer patients, with patients spending approximately 30% of their days seeking treatment [8, 10]. A distinctive U-shaped trajectory was observed by both Gupta and Johnson with a greater time toxicity following the initial diagnosis of metastatic cancer, and at the end of life [2, 10], which reflects the staging workup, multidisciplinary consultations in the beginning, and the demanding and increasing difficulty of symptom management towards the end of treatment. A matched, retrospective cohort study in Canada revealed that the degree of time toxicity is similar between more recent time and historic eras [11].
In this study, we summarized the methods and findings of the time toxicity studies in the oncology field (Table 1 [1, 2, 7, 10-18]). We reported the results of a retrospective study conducted in our general oncology clinic using a modified concept of treatment-related time toxicity (TrTT) to quantify the burden of time among individuals with advanced-stage cancers. We explored patterns between different cancers and treatment types, provided information to help guide clinicians in patient-centered discussions, and to ultimately decrease unnecessary disruption in patients’ lives.
 Click to view | Table 1. Summary of the Literature on Time Toxicity in Oncology |
| Materials and Methods | ▴Top |
A total of 150 patients who met the inclusion criteria were selected from a single-center, retrospective cohort. Data were collected via an electronic health record review under our institutional ethics guidelines. No identifiable patient information was collected. No in-person interviews or assessments were conducted. This study was conducted at the Ascension Seton Infusion Center, an oncology clinic site affiliated with the Dell Seton Medical Center at the University of Texas at Austin, TX, USA (the safety net hospital for Travis County) between January 1, 2021 and January 1, 2024. This study was conducted in compliance with the ethical standards of our institutional ethical guidelines. Institutional Review Board approval was not required for this type of study.
We selected individuals who received palliative treatment at our center for metastatic, incurable solid malignancies. Staging was based on the current National Comprehensive Cancer Network (NCCN) guidelines. Anonymous data, including cancer types, sociodemographic information, Eastern Cooperative Oncology Group (ECOG) performance status (PS), and types of cancer-directed therapies, were collected from the electronic health records. In-person health care contact records were counted and analyzed by our authors, including clinic visits, treatment visits, inpatient admissions, emergency department visits, laboratory tests, and radiology appointments. Telehealth visits and noncancer-related health care encounters were not included in the data collection. We restricted the analysis to the first 12 weeks from the initiation of the first line palliative therapy to minimize the chance for changes in therapy. We selected this initial 12-week window because it generally represents the most intensive treatment phase, during which patients face the highest density of visits and decisions regarding ongoing care.
The primary outcome was TrTT, defined as the number of days with any cancer-related health care encounters during the initial 12 weeks of palliative treatment. Days spent in the healthcare system not related to active cancer treatment were not included in this analysis (such as routine primary care visits, subspecialty visits, vaccinations, dental appointments, and social work visits). When two encounters occurred on the same date, we counted them as 1 day. We excluded patients with cancer of unknown primary, patients with stage IV cancer with oligometastasis who received curative surgical resection or curative radiotherapy, and patients who died within the 12-week period. Mild, moderate, and severe time toxicity were defined as < 10 days/12 weeks, 10 - 30 days/12 weeks, and > 30 days/12 weeks, respectively.
We stratified the TrTT by the type of cancer, type of systemic therapy received, and the ECOG PS. We plotted the total number of days per 12 weeks from starting palliative treatment using medians and interquartile ranges (IQRs). P values were calculated via the Kruskal-Wallis test for comparisons across more than two groups (e.g., different treatment types or cancer types), followed by post hoc pairwise Mann-Whitney tests when significant. The Graphpad Prism software was used for statistical analysis and plot generation.
| Results | ▴Top |
The median age of the 150 patients included in this study was 61 years (ranging from 23 to 86). Of the patients, 9.3% were older than 75 years, and 52.7% were biological male. Hispanic patients comprised 53% of the total population. The majority of patients had an ECOG score of 0 - 2 (74.6%). The sociodemographic and clinical characteristics are summarized in Table 2.
 Click to view | Table 2. Sociodemographic and Clinical Characteristics of the Patients |
The TrTT was highly variable among individuals. While scheduled office/treatment visits are fairly standardized and predictable, hospital visits varied greatly depending on the type of cancer, tumor burden, and ECOG PS. The median TrTT was 13 days/12 weeks (IQR 7 - 23). The majority of our patients experienced mild to moderate time toxicity (39% and 46%, respectively), with 14% experiencing severe toxicity, as defined by spending more than 1 out of 3 days pursuing treatment.
The type of treatment and cancer affects time toxicity. We found that immunotherapy was associated with significantly less TrTT (median = 7 days, IQR 4 - 10) compared with traditional chemotherapy (median = 22 days, P < 0.0001), while targeted therapy (median TrTT = 8, P < 0.0001) and hormone therapy (median = 3, P < 0.0001) also have lower TrTT than chemotherapy (Fig. 1). Among different cancer types, gastrointestinal malignancies were associated with the highest time toxicity with a median TrTT of 23 days (IQR 18 - 35) when compared with breast, thoracic, or genitourinary cancers (Fig. 1, P < 0.05).
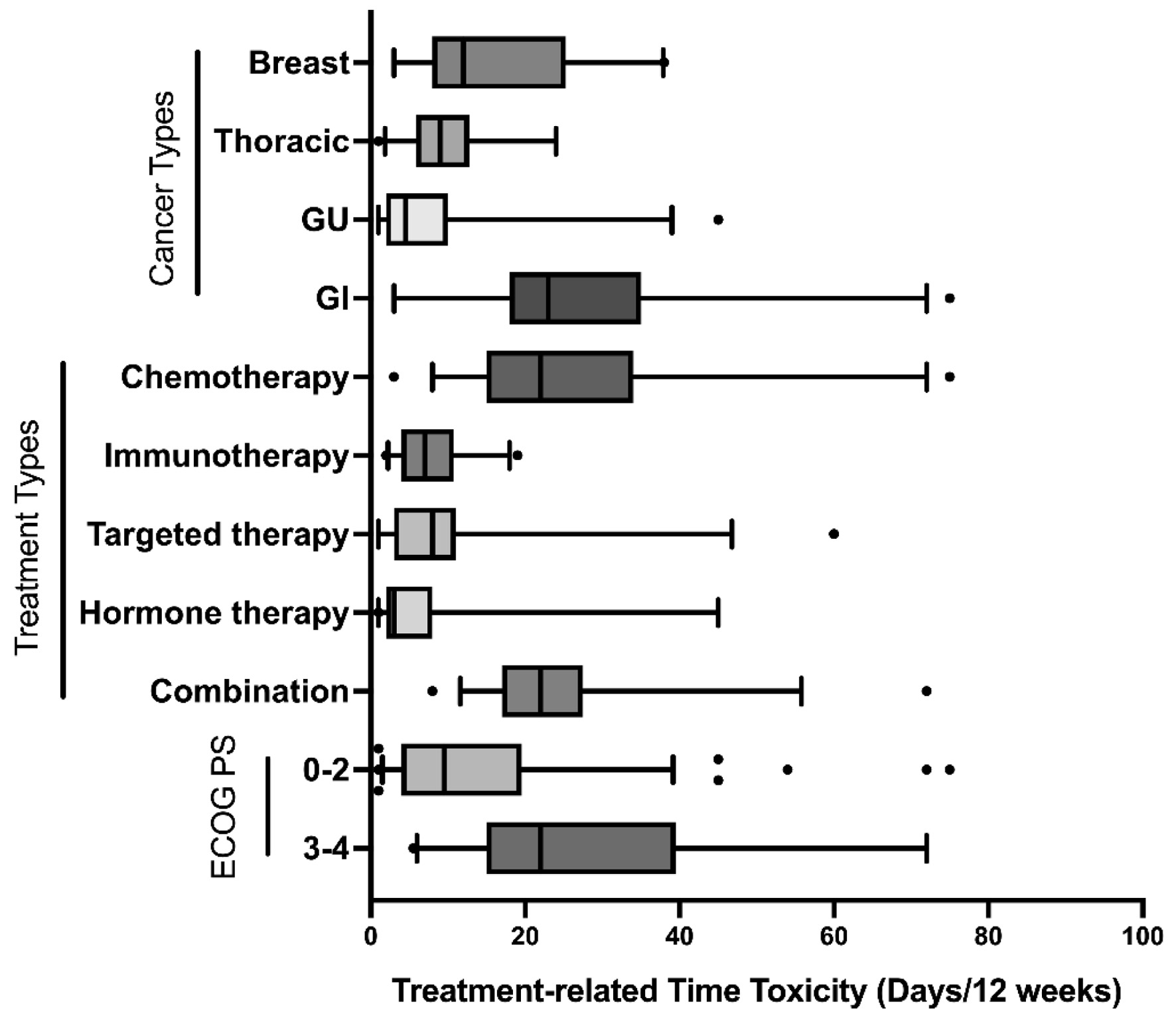 Click for large image | Figure 1. TrTT by subgroup. TrTTs were plotted on the basis of the cancer type, treatment type, or ECOG performance score with IQRs (box, between the 25% and 75% quartile value), median (lines within the box), 5th and 95th percentiles (whiskers), and outliers (dots). ECOG: Eastern Cooperative Oncology Group; IQR: interquartile range; TrTT: treatment-related time toxicity. |
Most oncology clinical trials include only patients with ECOG PS scale ranging from 0 to 2, as defined as those who are capable of all self-care and ambulatory more than 50% of waking hours. In our study, 25.4% of patients had ECOG PS from 3 to 4 (unable to perform full selfcare). These patients experienced more than double the TrTT than those with ECOG PS of 0 - 2 (median TrTT 22 vs. 9.5, P < 0.0001, Table 2). The differences in time toxicity between different subgroups are visualized in Figure 1 using the box and whisker plot.
| Discussion | ▴Top |
In this article, we present a modified concept of the TrTT as burden of time related to active and palliative cancer treatment, excluding time consumption that is not associated with cancer care, such as healthcare visits for other subspecialties or other comorbidities, dental visit, and routine vaccination. The TrTT is a more accurate metric for quantifying the negative impact of time in the palliative treatment setting. In our regional oncology clinic, 14% of patients with incurable advanced stage solid malignancies experienced severe TrTT. Newer generations of cancer treatments (immunotherapy, targeted therapy, and hormonal therapy) are associated with significantly lower time toxicity than chemotherapy. Patients with poorer PS or with gastrointestinal malignancies face higher time toxicity. Our findings are largely consistent with the published literature. For example, Baltussen et al demonstrated that older patients with metastatic cancer spend 1 in 5 days with healthcare contact during the first 6 months of treatment, with greater time toxicity associated with radiotherapy, chemotherapy, and high frailty [1]. The study design, patient population, and key findings from prior articles on time toxicity in oncology are summarized in Table 1.
There is a noticeable and alarming trend of using progression-free survival (PFS) as the primary endpoint in modern oncological phase III trials [19]. The percentage of trials using PFS as the primary endpoint in oncology climbed exponentially from 0% to 42% (from 1995 to 2009) [20]. These parameters are defined as the time from intervention to an event, such as disease progression on scans or death. PFS does not always reflect what truly matters to patients living with advanced cancer. The value of therapy often lies not solely in prolonging life, but also in preserving quality, time, and independence, especially for individuals living with incurable diseases. Many US Food and Drug Administration (FDA)-approved oncology interventions are based on small PFS benefits without any significant improvement in overall survival [19, 20]. The advantages of utilizing such approach are obvious: faster outcomes often lead to the acceleration of drug approvals. While PFS is an important factor to consider, many other components, including time toxicity, need to be considered to guide individualized decision making and provide meaningful clinical benefit to patients. Gupta et al proposed the “home day” metric as a simple way to measure meaningful treatment benefit, as identified as total survival days minus time toxicity [4]. Future clinical trials should consider including quality-adjusted survival benefits in their study design. The majority of patients declined treatments that offered only PFS benefits without overall survival gains when given hypothetical choices in the palliative oncology setting, especially when additional toxicity was expected [21-23]. These studies did not involve time toxicity discussion. One could extrapolate that the added time toxicity would make the proposed treatment choice even less attractive. In real-life scenarios, patient preferences are heavily influenced by physicians’ recommendations and their degrees of understanding of the realistic risk and benefit. Time toxicity should be included in these conversations. Time toxicity not only affects the patients, but also their caregivers and families. A total of 78% of patients in our clinic (calculated during a 1-week period) were accompanied by their caregivers. Patients with poorer PS often require external help for transportation and for keeping up with the complex oncology schedules.
We acknowledge that our findings should be interpreted in the context of several limitations. This study did not include patients undergoing clinical trials. Depending on the study design, clinical trials can generate significantly greater time toxicity due to more frequent visits, imaging scans, and longer commute to cancer centers. Our TrTT data are likely underestimated given that some but few of the hospital and emergency room visits were outside of our health system that were not available for collection. Notably, most of our patients did not have private insurance and utilized the county-funded medical access program; thus, a vast majority of these visits should be captured. Additionally, because we analyzed only the first 12 weeks of palliative therapy, our findings primarily capture the early, high-intensity phase of care. The time burden for patients receiving prolonged therapies such as maintenance immunotherapy or oral targeted agents may decrease over time as visit frequency becomes less intense, and future studies should investigate longitudinal changes in TrTT. Not all time toxicities are equally disruptive. A well-tolerated 6-h chemotherapy infusion session can be more pleasant than a 3-h emergency room visit for intractable vomiting. In an ideal world, we could assign severity to the time duration for a more accurate depiction of the time burden by patient-reported surveys. This retrospective data collection analysis was not designed to do so. Several studies were able to calculate the hours and minutes that patients spent per visit via objective measurements. Kagalwalla et al used real-time location system badge data to dissect clinic time into clinician, lab, infusion visits, and parking [7], which revealed that patients on average spent around 2 h per visit. While it is well recognized that patients with poor PS and those with gastrointestinal malignancies have higher symptom burden and health care utilization, our study quantifies this burden in a standardized manner, providing a metric that can be compared across populations and treatment modalities. We believe this quantitative approach may guide clinicians in balancing treatment intensity with quality-of-life considerations. Our sample size is modest but reflects a real-world safety-net population that is frequently underrepresented in clinical trials; these data may therefore be particularly relevant for practices caring for socioeconomically disadvantaged patients. Despite the inevitable limitations, we believe that our estimation provides valuable insights into the frequency of health care utilization.
Minimizing time toxicity requires changes in how oncology care is delivered. Small incremental improvements at each step of healthcare can lead to substantial gains in the quality of life for patients. When choosing between different types of therapies, time toxicity should be considered. Our study demonstrated that traditional chemotherapy posed significantly greater time toxicity when compared to immunotherapy, targeted therapy, or hormone therapy. This finding is consistent with our real-life experience, where chemotherapy tends to encompass more frequent visits, adverse effects, and hospitalizations. Prevention and management of aggressive adverse effects are crucial during cancer treatment. Patients with unaddressed side effects are more likely to seek urgent or emergent care, contributing further to unplanned time toxicity. To detect complications effectively, early symptom monitoring systems, such as through a patient-reported portal or regular nurse check-ins, are crucial and allow for outpatient rather than inpatient management. Technological advancements have transformed nearly every aspect of medicine. Telemedicine and home-based interventions reduce travel time and minimize disruption to a patient’s daily life. In our clinic, we implemented care-bundling to reduce time toxicity. In practice, this means scheduling pre-infusion laboratory tests on the morning of the infusion, followed immediately by the provider visit, and treatment in the same facility on the same day. This approach reduces the number of separate trips patients must make to the clinic and consolidates multiple encounters into one visit. Bange et al demonstrated that using digital text messaging tools can substantially decrease the burden of pre-infusion visits, saving patients over an hour per session without compromising safety [12]. Wearable monitoring devices, such as smartwatches and biosensors, have been proven to be useful in various oncology settings [24, 25]. Objective data from wearables related to sleep quality, physical activity, and caloric intake, can help oncologists monitor tolerance and compliance, and tailor treatment intensity. High risk changes in vital signs or symptoms can be flagged to allow early detection of conditions such as neutropenic fever, arrhythmias, and pulmonary embolism, and result in timely interventions and fewer hospital visits. The incorporation of artificial intelligence (AI) could provide powerful tools in time toxicity reduction. Cancellations and rescheduled appointments are especially common in the oncology offices. Fitting recurrent infusion visits of different lengths, frequencies, and durations are challenging tasks for the clinic staff. AI-powered scheduling systems can reduce wait times, improve translations and communications, handle scheduling changes, and optimize the infusion room utilization efficiency. By implementing strategies that preserve quality of life while minimizing disruptions, oncology providers can choose treatments that best align with the goals of their patients and their families. As cancer treatments continue to evolve rapidly, it is crucial that we consider the time toxicity of these new options.
Conclusion
TrTT is an important and often overlooked aspect of the overall treatment burden in oncology. As the complexity and duration of cancer treatments increase, addressing time toxicity becomes crucial to improving patients’ quality of life and overall outcomes. Our study revealed that severe time toxicity occurred in one in seven individuals with advanced stage solid malignancies, particularly in frail patients with gastrointestinal cancers or those receiving traditional chemotherapy. By recognizing and addressing time toxicity through better care coordination, telemedicine, and patient-centered approaches, the healthcare system can reduce this burden on cancer patients, allowing them to focus more on their goal of life and less on the logistical challenges of their treatment. Continued research and patient feedback will be essential in refining strategies to mitigate time toxicity and ensuring that the time demands of cancer treatment do not unduly compromise patients’ well-being.
Acknowledgments
The authors thank Dr. Kathleen T. Li (University of Texas, Austin, TX) for her expertise in statistical analysis and thoughtful editorial assistance.
Financial Disclosure
None to declare.
Conflict of Interest
All authors report no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Yuhang Zhou, Kyaw Aung, Om Pandey, and Boone Goodgame conceived and designed the analysis; Yuhang Zhou, Madeline Fitzpatrick, Sujata Ojha, Marisabel Hurtado-Castillo, and Williams Sessions collected the data; Yuhang Zhou and Madeline Fitzpatrick performed the analysis; Yuhang Zhou, Madeline Fitzpatrick, Sujata Ojha, Marisabel Hurtado-Castillo, Williams Sessions, Kyaw Aung, and Boone Goodgame wrote the paper.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
AI: artificial intelligence; CDC: Centers for Disease Control and Prevention; ECOG: Eastern Cooperative Oncology Group; FDA: US Food and Drug Administration; NCCN: National Comprehensive Cancer Network; PFS: progression-free survival; TrTT: treatment-related time toxicity
| References | ▴Top |
- Baltussen JC, Cardenas-Reyes P, Chavarri-Guerra Y, Ramirez-Fontes A, Morales-Alfaro A, Portielje JEA, Ramos-Lopez WA, et al. Time toxicity among older patients with cancer treated with palliative systemic therapy. Support Care Cancer. 2024;32(9):621.
doi pubmed - Gupta A, Nguyen P, Kain D, Robinson AG, Kulkarni AA, Johnson DH, Presley CJ, et al. Trajectories of health care contact days for patients with stage IV non-small cell lung cancer. JAMA Netw Open. 2024;7(4):e244278.
doi pubmed - Gupta A, Jensen EH, Virnig BA, Beg MS. Time-related burdens of cancer care. JCO Oncol Pract. 2022;18(4):245-246.
doi pubmed - Gupta A, Eisenhauer EA, Booth CM. The time toxicity of cancer treatment. J Clin Oncol. 2022;40(15):1611-1615.
doi pubmed - Prasad V, Olivier T, Chen EY, Haslam A. Estimation of time cost of anti-cancer drugs approved based on comparisons to best supportive care: A cross sectional analysis. J Cancer Policy. 2022;34:100363.
doi pubmed - Lim SA, Hao SB, Boyd BA, Mitsakos A, Irish W, Burke AM, Parikh AA, et al. Opportunity costs of surgical resection and perioperative chemotherapy for locoregional pancreatic adenocarcinoma. JCO Oncol Pract. 2022;18(4):302-309.
doi pubmed - Kagalwalla S, Tsai AK, George M, Waldock A, Davis S, Jewett P, Vogel RI, et al. Consuming patients' days: time spent on ambulatory appointments by people with cancer. Oncologist. 2024;29(5):400-406.
doi pubmed - Patel VR, Ramesh V, Tsai AK, Sedhom R, Westanmo AD, Blaes AH, Vogel RI, et al. Health care contact days experienced by decedents with advanced GI cancer. JCO Oncol Pract. 2023;19(11):1031-1038.
doi pubmed - Ioannidis JP, Pavlidis N. Levels of absolute survival benefit for systemic therapies of advanced cancer. a call for standards. Eur J Cancer. 2003;39(9):1194-1198.
doi pubmed - Johnson WV, Phung QH, Patel VR, Tsai AK, Arora N, Klein MA, Westanmo AD, et al. Trajectory of healthcare contact days for veterans with advanced gastrointestinal malignancy. Oncologist. 2024;29(2):e290-e293.
doi pubmed - Bateni SB, Nguyen P, Eskander A, Seung SJ, Mittmann N, Jalink M, Gupta A, et al. Changes in health care costs, survival, and time toxicity in the era of immunotherapy and targeted systemic therapy for melanoma. JAMA Dermatol. 2023;159(11):1195-1204.
doi pubmed - Bange EM, Coughlin K, Li W, Moriarty E, Brown TJ, Shulman LN, Mamtani R. Accuracy of a text intervention to minimize the burden of cancer care among patients treated with immune checkpoint inhibitors. JAMA Netw Open. 2022;5(8):e2228452.
doi pubmed - Guy GP, Jr., Richardson LC. Visit duration for outpatient physician office visits among patients with cancer. J Oncol Pract. 2012;8(3 Suppl):2s-8s.
doi pubmed - Ray KN, Chari AV, Engberg J, Bertolet M, Mehrotra A. Disparities in time spent seeking medical care in the United States. JAMA Intern Med. 2015;175(12):1983-1986.
doi pubmed - Rocque GB, Williams CP, Ingram SA, Azuero A, Mennemeyer ST, Young Pierce J, Nipp RD, et al. Health care-related time costs in patients with metastatic breast cancer. Cancer Med. 2020;9(22):8423-8431.
doi pubmed - Gupta A, O'Callaghan CJ, Zhu L, Jonker DJ, Wong RPW, Colwell B, Moore MJ, et al. Evaluating the time toxicity of cancer treatment in the CCTG CO.17 trial. JCO Oncol Pract. 2023;19(6):e859-e866.
doi pubmed - Gupta A, Chant ED, Mohile S, Vogel RI, Parsons HM, Blaes AH, Booth CM, et al. Health care contact days among older cancer survivors. JCO Oncol Pract. 2024;20(7):943-952.
doi pubmed - Banerjee R, Cowan AJ, Ortega M, Missimer C, Carpenter PA, Oshima MU, Salit RB, et al. Financial toxicity, time toxicity, and quality of life in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2024;24(7):446-454.e443.
doi pubmed - Walia A, Tuia J, Prasad V. Progression-free survival, disease-free survival and other composite end points in oncology: improved reporting is needed. Nat Rev Clin Oncol. 2023;20(12):885-895.
doi pubmed - Del Paggio JC, Berry JS, Hopman WM, Eisenhauer EA, Prasad V, Gyawali B, Booth CM. Evolution of the Randomized Clinical Trial in the Era of Precision Oncology. JAMA Oncol. 2021;7(5):728-734.
doi pubmed - Brundage MD, Booth CM, Eisenhauer EA, Galica J, Kankesan J, Karim S, Koven R, et al. Patients' attitudes and preferences toward delayed disease progression in the absence of improved survival. J Natl Cancer Inst. 2023;115(12):1526-1534.
doi pubmed - Robinson AG, O'Donnell J, Booth C, Koven R, Eisenhauer E, Brundage M. Patient perspectives of value of delayed disease progression on imaging (imaging PFS). A treatment trade-off experiment. J Cancer Policy. 2021;30:100301.
doi pubmed - Raphael MJ, Robinson A, Booth CM, O'Donnell J, Palmer M, Eisenhauer E, Brundage M. The value of progression-free survival as a treatment end point among patients with advanced cancer: a systematic review and qualitative assessment of the literature. JAMA Oncol. 2019;5(12):1779-1789.
doi pubmed - Hunter H, Bennington-McKay N, Sher J, Psutka SP, Lin C. Emerging role of mobile applications and wearable devices for prehabilitation in urologic oncology. Eur Urol Focus. 2024;10(1):20-22.
doi pubmed - Chow R, Drkulec H, Im JHB, Tsai J, Nafees A, Kumar S, Hou T, et al. The use of wearable devices in oncology patients: a systematic review. Oncologist. 2024;29(4):e419-e430.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.