| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 4, August 2025, pages 331-341
Efficacy of Infliximab Versus Vedolizumab in the Management of Immune Checkpoint Inhibitor-Induced Colitis: A Systematic Review and Meta-Analysis
Shreya Shambhavia, g, Harmanjeet Singhb, Ganesh Ramaprasadc, Murod Khikmatovd, Varun Vankeshwarama, Kumar Ashishe, Carlos Valladaresa, Tanushree Bhattf, Seth D. Cohena
aRutgers Health/Community Medical Center, Toms River, NJ, USA
bMahatma Gandhi Memorial Medical College, Jamshedpur, Jharkhand, India
cMary Washington Healthcare, Fredericksburg, VA, USA
dDepartment of Medicine, Rowan-Virtua University School of Osteopathic Medicine, Stratford, NJ 08084, USA
eNazareth Hospital, Trinity-Health Mid-Atlantic, Philadelphia, PA, USA
fHenry Ford St. John Hospital, Detroit, MI, USA
gCorresponding Author: Shreya Shambhavi, Rutgers Health/Community Medical Center, Toms River, NJ, USA
Manuscript submitted May 26, 2025, accepted July 10, 2025, published online July 26, 2025
Short title: IFX vs. VDZ in the Management of ICI-Induced Colitis
doi: https://doi.org/10.14740/wjon2613
| Abstract | ▴Top |
Background: Immune checkpoint inhibitors (ICIs) can cause severe gastrointestinal immune-related adverse events (irAEs), often leading to treatment interruption and increased morbidity. Immune-mediated colitis (IMC) ranges from mild diarrhea to life-threatening colitis, sometimes requiring urgent intervention. While corticosteroids are the first-line treatment, selective immunosuppressive therapy (SIT) with either infliximab or vedolizumab is used for steroid-refractory or dependent cases. However, standardized practices are lacking, and treatment decisions are largely left to provider discretion. This study compares infliximab and vedolizumab for IMC, focusing on remission rates, recurrence, SIT dosing, and systemic steroid exposure duration.
Methods: We identified six retrospective cohort studies that compared infliximab with vedolizumab in the treatment of IMC through a systematic search of PubMed, EMBASE, Cochrane Library, Scopus, CINAHL, Google Scholar, and Web of Science in English from inception until October 2024. From the identified literature, we extracted pertinent data such as remission and recurrence of IMC. Pooled analysis and heterogeneity analysis were performed using R Studio version 4.4.1. The risk of bias was assessed using the Newcastle-Ottawa Scale.
Results: A total of six studies with 645 patients were included. In ICI-associated colitis, vedolizumab was associated with lower recurrence rates (odds ratio (OR): 0.29, 95% confidence interval (CI): 0.15 - 0.54) and shorter systemic steroid exposure (mean difference (MD): -16.88 days, 95% CI: -20.47 to -13.30) compared to infliximab. While vedolizumab showed improved remission, there was no statistically significant difference in remission rates between vedolizumab and infliximab monotherapy (OR: 3.16, 95% CI: 0.29 - 34.01). Remission was achieved with fewer doses of infliximab than vedolizumab (MD: 1.16, 95% CI: 0.09 - 2.22). The mean number of vedolizumab doses was 2.57 (raw mean score (MRAW): 2.57, 95% CI: 1.43 - 2.71), while the mean number of infliximab doses was 1.36 (MRAW: 1.36, 95% CI: 0.69 - 2.02).
Conclusions: Among patients with ICI-induced colitis, vedolizumab demonstrated superiority over infliximab by being associated with lower rates of colitis recurrence and decreased systemic steroid exposure, although it required a higher number of doses compared to infliximab.
Keywords: Vedolizumab; Immune-mediated colitis; Infliximab; Steroids; PDL-1; CTLA-4
| Introduction | ▴Top |
Immune checkpoint inhibitors (ICIs) are innovative cancer therapies that enhance the immune system by targeting pathways that suppress T-cell activity. Targets include cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), which, after expression on T cells, competes with CD28 to bind to B7 ligands, thus preventing the costimulatory signals required for full T-cell activation [1]. Alternatively, programmed death-1 (PD-1) binding with programmed death ligand-1 (PD-L1) and programmed death ligand-2 (PD-L2) expressed on certain malignant cells suppresses T-cell receptor signaling [2]. By blocking these pathways, ICIs restore T-cell antitumor activity, improving outcomes in various cancers [3]. These therapies revolutionized cancer management and improved overall survival in various malignancies that include lung, head, neck, and gastroesophageal cancers, and they benefited patients with reduced recurrence rates. Immune checkpoint proteins are crucial for peripheral tolerance, and their blockade can result in autoimmune reactions, also referred to as immune-related adverse effects (irAEs), which is why using ICIs could result in complications [4]. The mechanism likely involves sustained T-cell activation caused by ICIs [5]. ICIs are associated with severe gastrointestinal (GI) irAEs, often requiring treatment pauses and withdrawal to improve morbidity and mortality [6]. Immune-mediated colitis (IMC) is defined as the development of diarrhea (increase in stool frequency over baseline), abdominal pain, rectal bleeding, urgency, mucus or blood in stool, and/or fever after initiation of ICIs. It is mainly associated with CTLA-4 therapy, has an incidence of 5.7-39.1% with CTLA-4 inhibitors, and 0.7-31.6% with PD-1/PD-L1 inhibitors; combination therapy can raise this to 40.4% [6, 7]. IMC symptoms range from mild diarrhea to severe colitis, requiring urgent intervention within 6 to 8 weeks of immunotherapy to prevent complications like colonic perforation or sepsis [5, 6]. Interestingly, irAEs, including IMC, correlate with enhanced therapeutic efficacy, making management crucial for patient outcomes [8]. The treatment for IMC typically involves corticosteroids [9]. However, tumor necrosis factor-alpha (TNF-A) inhibitors like infliximab (IFX), which inhibit neutrophil tissue migration, are considered when patients are steroid-refractory without significant improvement [10]. Vedolizumab (VDZ), a gut-selective α4β7 integrin antagonist, offers an alternative treatment modality by targeting GI homing T lymphocytes [11]. Both therapies, known as selective immunosuppressive therapies (SIT), have shown promising results in treating IMC [12, 13]. However, there are no standardized practices, and mostly, provider discretion is used in the treatment of IMC. This meta-analysis compares VDZ versus (vs) IFX for IMC to determine if either therapy offers superior clinical outcomes in terms of remission and recurrence of IMC or duration of exposure to systemic steroids.
| Materials and Methods | ▴Top |
The present systematic review followed the recommendations and criteria established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines [14]. The protocol was pre-registered at the International Prospective Register of Systematic Review (PROSPERO) with the identifier code CRD420250608615. After developing the clinical question and translating it into a well-defined systematic review question based on the PICOS format (Patients, Interventions, Comparators, Outcomes, and Studies), a manual search of medical databases was performed, including PubMed, EMBASE, Cochrane Library, Scopus, CINAHL, Google Scholar, and Web of Science in English from inception until October 2024. The search was conducted using the following PICO format: P: adult participants (age > 18 years) with ICI-mediated colitis; I: intervention group treated with VDZ; C: control group treated with IFX; O: remission of colitis; S: observational studies.
Eligibility criteria
We performed a systematic review of English-language studies in the database from inception through October 2024, including cohort studies, case-control studies, and case series (with > 5 patients). Studies were included if: 1) They involved humans; 2) ≥ 18-year-old participants were enrolled; 3) Patients who developed colitis after receiving one or more doses of ICI therapies (CTLA-4 or PD-1/PD-L1 inhibitors) and who were treated with selective immunosuppression therapy with either VDZ or IFX monotherapy or sequential IFX followed by VDZ therapy apart from systemic steroids; and 4) Among all the results, at least one of the following outcomes was available or could be calculated: a) Recurrence of colitis, which was defined as return of IMC symptoms (e.g., diarrhea, abdominal pain) after initial resolution, requiring renewed medical intervention; b) Remission of colitis, defined as sustained resolution of diarrhea or colitis to grade 1 or lower; c) median duration of steroid exposure in the treatment of colitis with each SIT; and d) Difference in mean SIT doses between VDZ and IFX. Studies were excluded if: 1) They were non-human studies (cell culture, animal models); 2) Case reports, book chapters, protocol articles, reviews, news articles, letters to the editor, editorials; 3) They were duplicates; 4) These studies were published in non-English languages or lacked English-translated versions; 5) The data were incomplete on clinical outcome statistical measures (odds ratios (ORs), 95% confidence interval (CI)), or the outcome measures were unable to be calculated with the available data; or 6) Studies in which VDZ or IFX was primarily used in treatment of other types of colitis apart from IMC, such as ulcerative colitis, Crohn’s disease, microscopic colitis, infectious colitis and ischemic colitis.
Outcome measures
The outcomes of this systematic review and meta-analysis were remission of IMC, recurrence of IMC, and median duration of steroid exposure with either VDZ or IFX monotherapy.
Data synthesis and analysis
The selected studies were reviewed, and the data were independently extracted by two researchers (SS and HS). Discrepancies between them were resolved by the third researcher, GR. Data were extracted from the eligible articles as follows: author, publication year, country/region, study type, sample size, age, and outcomes. Data were imported into a bibliographic database using Microsoft® Excel® 2022 (Microsoft, Redmond, WA, USA).
We conducted a narrative synthesis of the study results, structured around the target population’s characteristics and their exposure. The effect measures for the outcomes were calculated using R Studio version 4.4.1. ORs with 95% CIs were used for analysis when available. If not provided, crude estimates were calculated from 2 × 2 contingency tables. Differences were considered statistically significant when P < 0.05. Study heterogeneity was assessed using the I2 statistic, quantifying the percentage of variation attributable to between-study differences, per Cochrane Handbook guidelines [15]. The I2 values were interpreted as follows: < 30% indicated low heterogeneity, 30-60% moderate heterogeneity, 60-75% substantial heterogeneity, and > 75% considerable heterogeneity. Using the DerSimonian and Laird method, a random-effects meta-analysis was performed to estimate the pooled OR and 95% CI [16]. Since this study was a meta-analysis of published studies, the Institutional Review Board approval was not required.
Ethical compliance
Since this is a meta-analysis of already published studies, no comments can be made on ethical animal/human rights. The included studies were ethically compliant to animal/human safety.
Search results
A total of 8,847 articles were collected from seven database searches. Before screening, 1,418 duplicate articles were removed. From the remaining 7,429 records, 7,332 were excluded during the screening process. Of the remaining publications, 97 were sought for retrieval, out of which 36 reports could not be retrieved. A total of 61 articles underwent full-text review for eligibility. We excluded 30 studies with incorrect methodology and 13 more that did not satisfy the population criteria. After excluding 12 more articles for inappropriate publication formats, six retrospective cohort studies remained and were included in our analysis. Table 1 summarizes the characteristics of the included studies. Table 2 presents the outcomes of interest reported for each study, detailing the various types of ICIs administered and the number of individual SIT doses given. The systematic approach adopted for the study selection is illustrated in Figure 1 of the PRISMA flow diagram. The detailed search strategy with yielded results is summarized here (Supplementary Material 1, wjon.elmerpub.com). Figure 2 depicts the overall risk of bias in the included studies.
 Click to view | Table 1. Characteristics of Studies Included |
 Click to view | Table 2. Number of Patients Receiving Particular ICI and Number of Doses of SIT Administered |
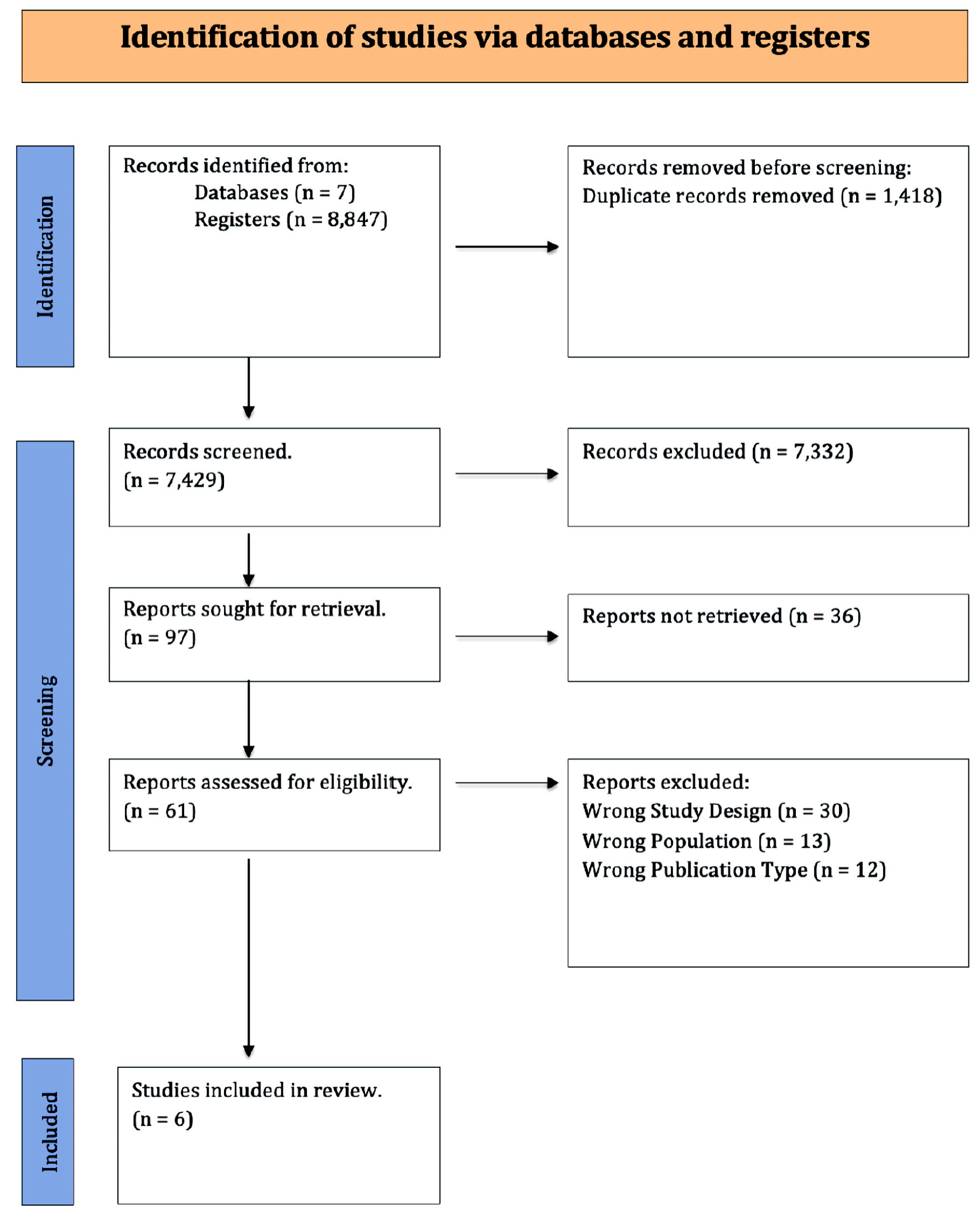 Click for large image | Figure 1. PRISMA flowchart. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
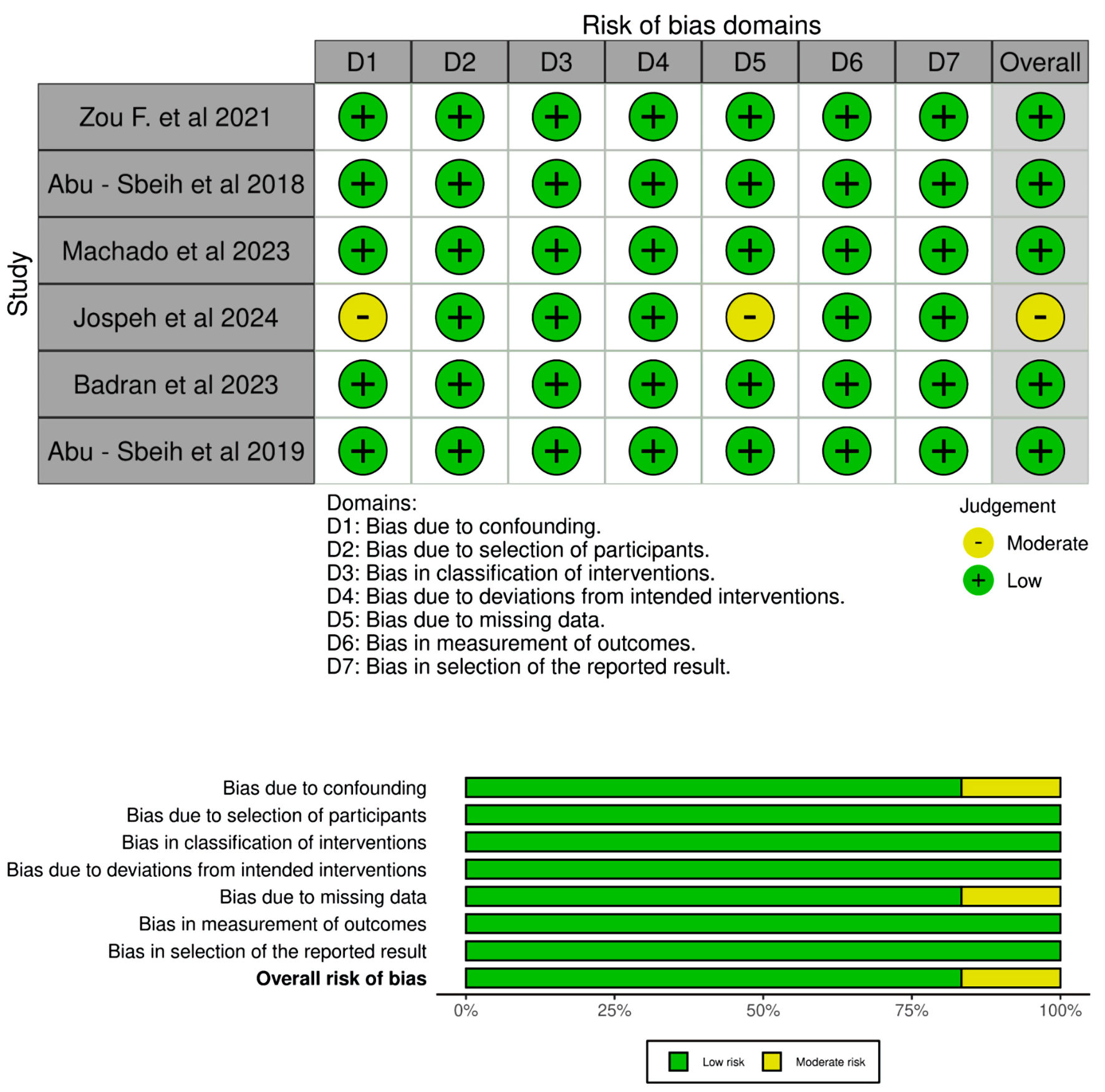 Click for large image | Figure 2. Risk of bias assessment of selected studies. |
Study quality assessment
The quality of the observational studies was assessed using the Newcastle-Ottawa Scale, which evaluates three domains: patient selection, comparability of study groups, and outcome assessment [17]. Studies were categorized as high quality (7 - 9 points), moderate quality (4 - 6 points), or low quality (≤ 3 points). Two investigators (SS and HS) independently assessed the risk of bias, with any discrepancies resolved through consensus by all authors.
| Results | ▴Top |
Recurrence
Patients with IMC who were treated with either IFX or VDZ and/or who resumed ICI with concomitant IFX or VDZ were included in the analysis for the recurrence of colitis after SIT treatment. Analysis of recurrence was conducted on 311 patients across three studies (138 VDZ vs. 173 IFX) [18-20]. The pooled OR indicated that the VDZ group had a significantly reduced risk of recurrence of colitis as compared to the IFX group (OR: 0.29, 95% CI: 0.15, 0.54) with no statistically significant heterogeneity (I2 = 0%, P = 0.47), as described in Figure 3.
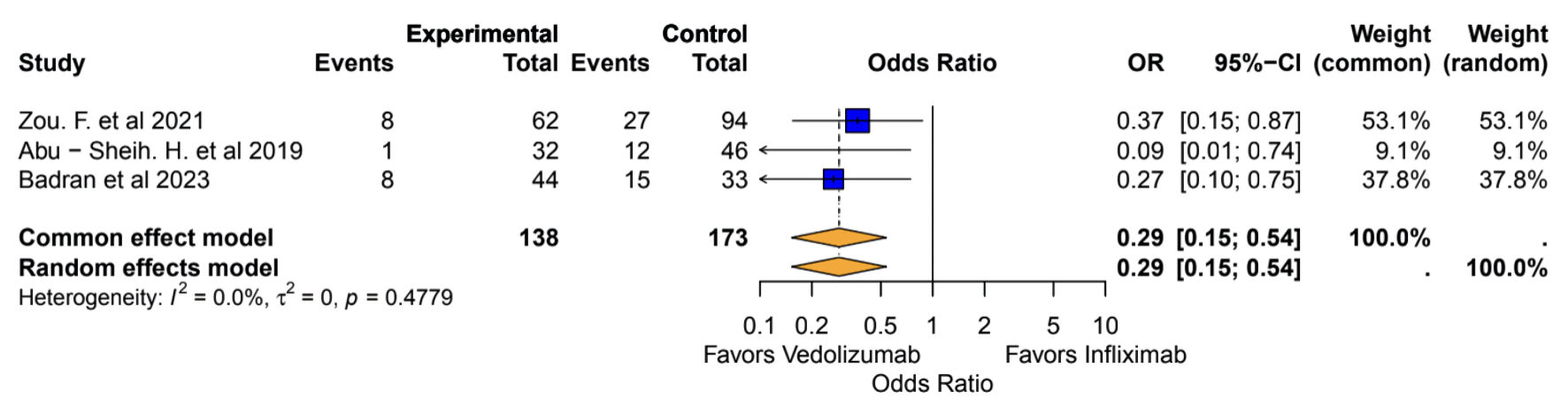 Click for large image | Figure 3. Recurrence of colitis: vedolizumab vs. infliximab. OR: odds ratio; CI: confidence interval. |
Median duration of steroids
All patients with ICI-mediated colitis who were treated with either IFX monotherapy or VDZ monotherapy, apart from systemic corticosteroids, were included in the final analysis. Analysis of the median duration of steroid exposure was conducted on 351 patients across three studies (143 VDZ vs. 208 IFX) [18, 21, 22]. The pooled mean difference (MD) indicated that the VDZ group had a lower overall median steroid exposure in comparison to the IFX monotherapy group (MD: -16.88, 95% CI: -20.47, -13.30) with no heterogeneity (I2 = 0%, P = 0.40), as described in Figure 4.
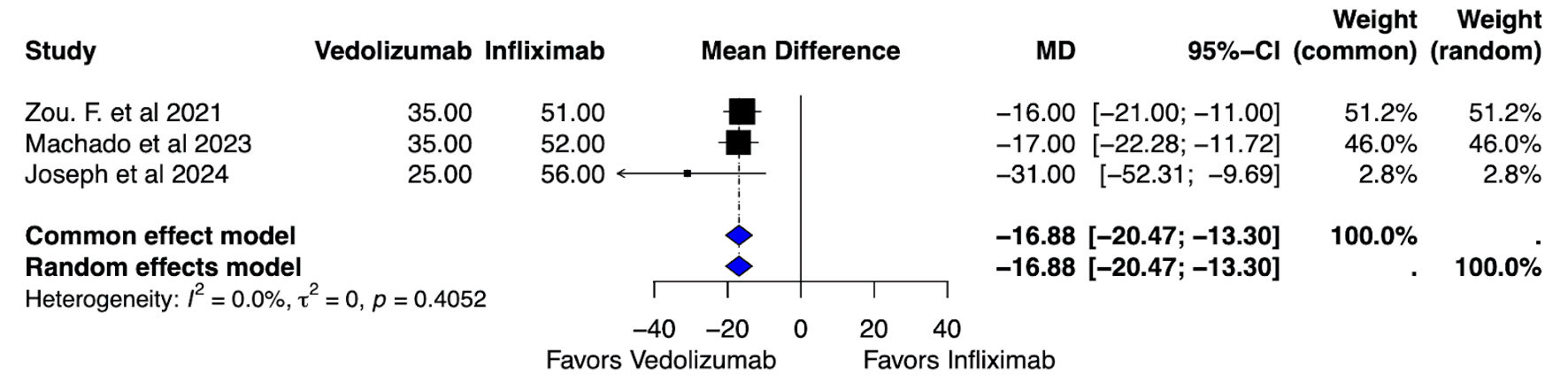 Click for large image | Figure 4. Median dose of steroid exposure: vedolizumab vs. infliximab. MD: mean difference; CI: confidence interval. |
Remission
Patients with ICI-mediated colitis who were treated with IFX and then followed by VDZ due to a lack of optimal response with IFX alone were included in the efficacy evaluation for remission. Analysis of remission was conducted on 239 patients across three studies (103 VDZ vs. 136 IFX) [18, 22, 23]. The pooled OR indicated that VDZ showed higher rates of remission as compared to IFX, although it was not statistically significant (OR = 3.16, 95% CI: 0.29, 34.01) and had considerable heterogeneity (I2 = 80.8%, P = 0.005), as described in Figure 5.
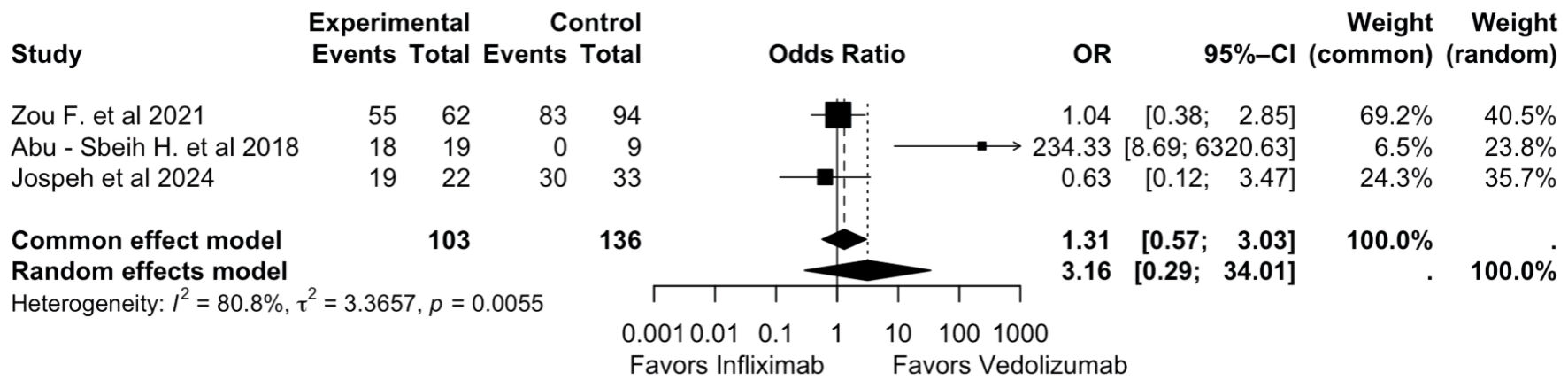 Click for large image | Figure 5. Remission of colitis: vedolizumab vs. infliximab. OR: odds ratio; CI: confidence interval. |
Difference in mean SIT doses
All patients with ICI-mediated colitis who were treated with either IFX or VDZ, along with systemic corticosteroids, were included in the final quantitative analysis. Analysis of the median duration of steroid exposure was conducted on 472 patients across five studies (205 VDZ vs. 267 IFX) [18, 19, 21-23]. The pooled MD indicated that remission was achieved with fewer doses of IFX than with VDZ (MD: 1.16, 95% CI: 0.09, 2.22), with considerable heterogeneity (I2 = 90.3%, P <0.001), as described in Figure 6.
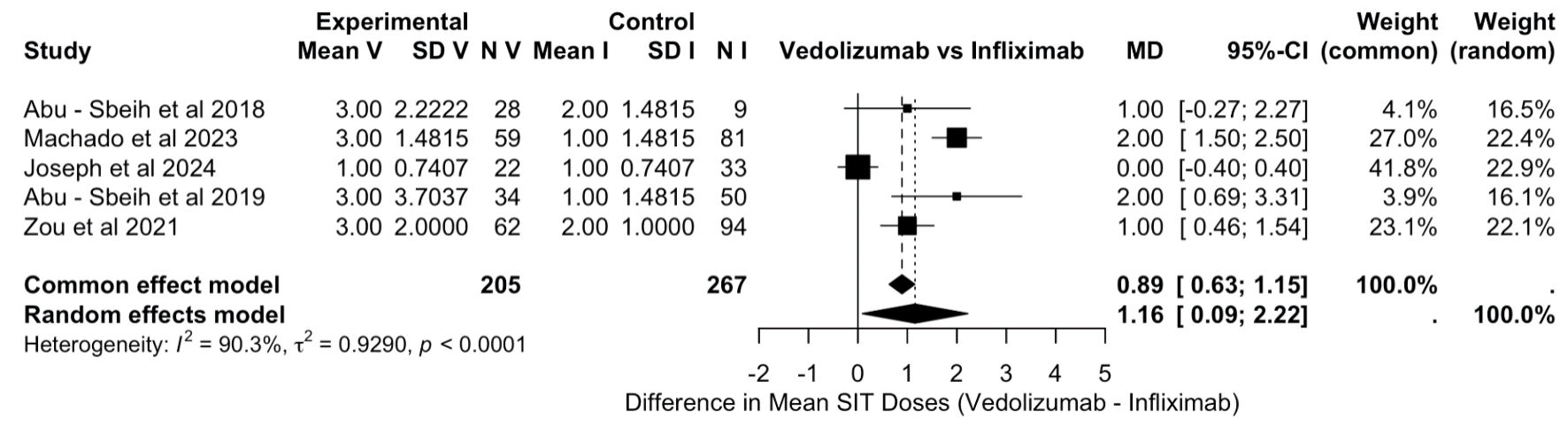 Click for large image | Figure 6. Difference in mean SIT doses (vedolizumab vs. infliximab). SD: standard deviation; MD: mean difference; CI: confidence interval. |
Mean doses of VDZ
All patients with ICI-mediated colitis who were treated with VDZ, along with systemic corticosteroids were included in the final analysis. Analysis of the mean doses of VDZ was conducted on 205 patients across five studies [18, 19, 21-23]. The pooled mean number of VDZ doses required was 2.57 (raw mean score (MRAW): 2.57, 95% CI: 1.43, 2.71), with significant heterogeneity (I2 = 95.5%), as described in Figure 7.
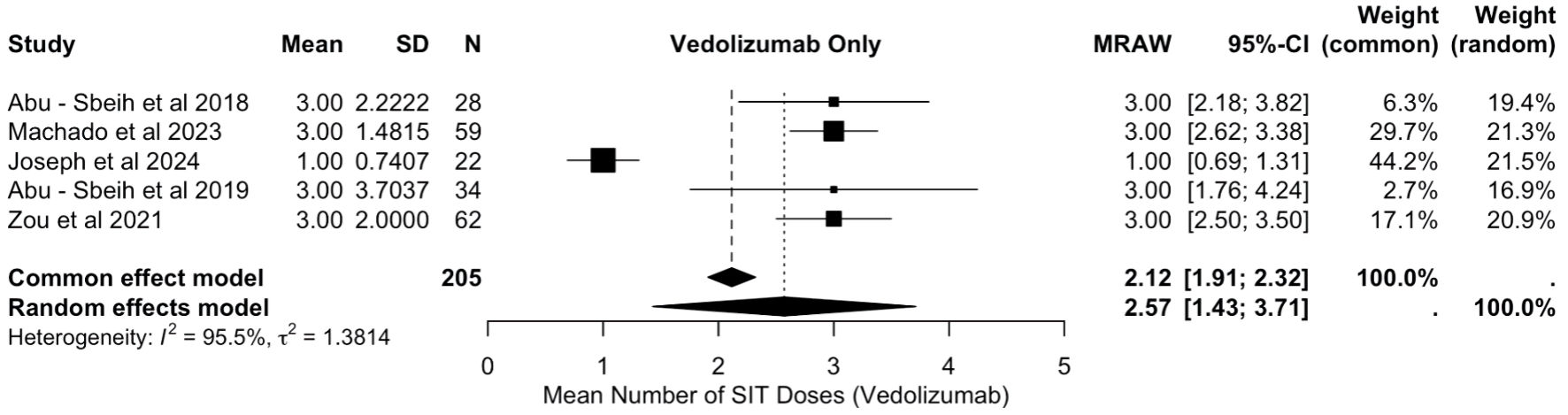 Click for large image | Figure 7. Mean doses of vedolizumab. SD: standard deviation; MRAW: raw mean score; CI: confidence interval. |
Mean doses of IFX
All patients with ICI-mediated colitis who were treated with VDZ, along with systemic corticosteroids were included in the final analysis. The analysis of mean IFX doses included data from 267 patients across five studies [18, 19, 21-23]. The pooled mean number of IFX doses required was 1.36 (MRAW: 1.36, 95% CI: 0.69, 2.02), with considerable heterogeneity (I2 = 92.6%), as shown in Figure 8.
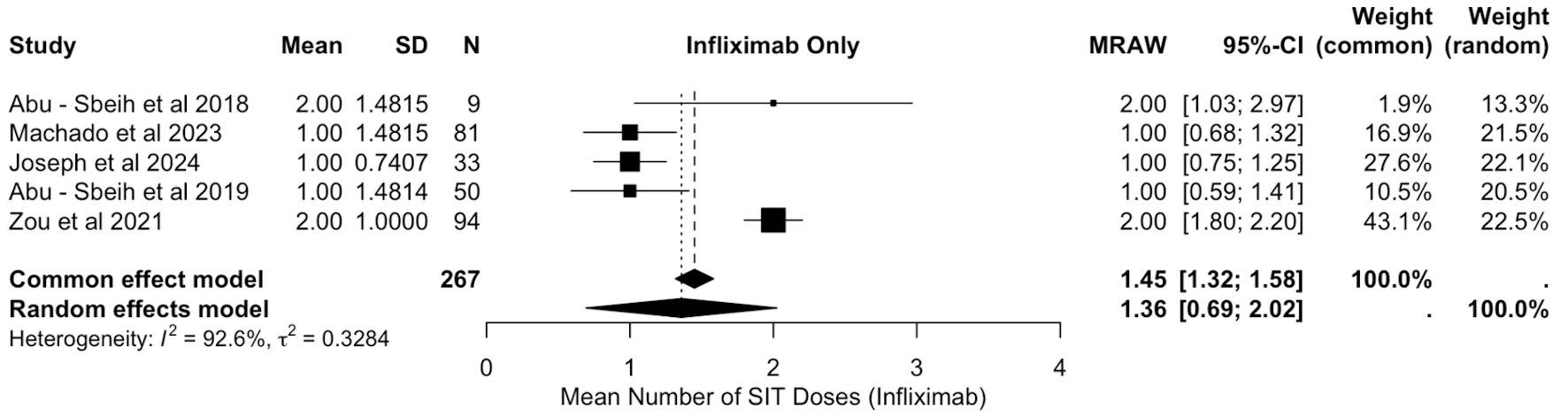 Click for large image | Figure 8. Mean doses of infliximab. SIT: selective immunosuppressive therapy; SD: standard deviation; CI: confidence interval; MRAW: raw mean score. |
| Discussion | ▴Top |
This analysis is the first and most comprehensive to compare and explore the selective immunosuppressant treatment modalities for ICI-mediated colitis. Established algorithms have corticosteroids as the mainstay treatment for ICI-mediated colitis [24]. When patients are either steroid-refractory or steroid-resistant, IFX, a monoclonal antibody targeting TNF-A, has been more commonly used as the selective immunosuppressant agent [25]. On the contrary, VDZ is a less studied and more recently approved humanized antibody that inhibits gut lymphocyte trafficking, hence enabling selective GI immunosuppression [26, 27]. Because of its gut tropism, it might have a reduced overall influence on the effect of cancer development and treatment. Also, it is not associated with the adverse effects related to IFX, which include potential reactivation of serious infections like latent tuberculosis, exacerbation of underlying heart failure, hepatotoxicity, and increased risk of autoimmune diseases and lymphoma. Our meta-analysis concludes that patients treated with VDZ had lower rates of recurrence of colitis as compared to patients on IFX (OR = 0.29, 95% CI: 0.15, 0.84). This can help prevent ICI cessation after its resumption, as IMC recurrence is one of the common causes of ICI failure. Our analysis also demonstrated that VDZ is associated with a lesser duration of steroid exposure when compared to IFX (MD: -16.88, 95% CI: -20.47, -13.30). Reduced exposure to steroids is beneficial for patients suffering from cancer, as the overall duration of steroid exposure was associated with worse survival in univariate analysis, an observation that has been made in studies of other irAEs as well [28, 29]. Also, systemic corticosteroids are strongly associated with serious infections, and the risk increases significantly with the addition of systemic immunosuppressants. Prior studies have shown a high rate of serious infections with steroids and IFX identified as the main risk factors [30-33]. The dosage for corticosteroids was based on the standard Common Terminology Criteria for Adverse Events (CTCAE) grade-based American Society of Clinical Oncology (ASCO) guidelines for ICI-induced colitis, with 1 mg/kg/day prednisone being the recommended initial dose of steroid for grade 2 IMC, and 1 - 2 mg/kg/day being the recommended initial dose of steroid for grade 3-4 IMC. Most studies also reported that it was prudent to calculate the median duration of corticosteroid exposure, but impractical to calculate the cumulative dose as it varied depending on the grade of IMC. But the steroid dose between the participants of either group (VDZ or IFX) were pretty balanced. This is because SIT was incorporated mostly when patients were deemed either steroid refractory/steroid dependent after they had received the highest dose of corticosteroids, which was 2 mg/kg/day prednisone or equivalent. Studies of IFX have focused on its safety in non-cancer populations, with limited evidence guiding its use in cancer patients with colitis [34]. Reactivation of tuberculosis has been reported in cancer patients receiving ICIs, and the concurrent use of IFX may further increase this risk. So, VDZ should be considered to treat ICI-induced colitis in patients with a contraindication for anti-TNFα agents, such as latent tuberculosis or hepatitis B. On the contrary, VDZ, even as a long-term treatment, seems to have a favorable safety profile in cancer patients [18]. Although, a quantitative analysis could not be performed because infection data for both IFX and VDZ were reported in only two out of the six included studies, the favorable safety profile of VDZ has been linked to its gut-selective mechanism of action, resulting in lower rates of infections (25% with IFX vs. 19% with VDZ, as reported in one major study, Zou et al, 2021), reduced systemic immunosuppression, and a decreased risk of cancer progression (53% with IFX vs. 34% with VDZ) [18].
Moreover, VDZ offers a fixed-dose regimen (300 mg intravenous (IV)) in comparison with weight-based dosing of IFX (5 mg/kg initially, with potential escalation to 10 mg/kg for loss of response) streamlining administration and reducing dosing errors. According to current guidelines, SIT is administered with VDZ at a dose of 300 mg intravenously, or with IFX at 5 mg/kg given on day 1, followed by a second infusion 2 weeks later, a third dose after 6 weeks, and then every 8 weeks as needed thereafter. Our quantitative analysis of doses of IFX revealed a pooled IFX dose of 1.36 (0.69 - 2.02), while the quantitative analysis of doses of VDZ revealed a pooled VDZ dose of 2.57 (1.43 - 3.71). This indicates that, on average, IFX achieves remission with up to two doses, whereas VDZ typically requires up to three doses for optimal clinical response. Among the included studies, Zou et al (2021) reported median duration to symptom remission of 13 days (IQR: 8 - 29) for IFX and 18 days (IQR: 10 - 40) for VDZ. Abu-Sbeih et al (2018) found a median of 5 days (IQR: 1 - 30) for VDZ, while Joseph et al (2024) reported 10 days for IFX and 18 days for VDZ [18, 22, 23]. Overall, the average time to improvement of IMC to grade 1 was about 11.5 days for IFX and 13.7 days for VDZ, though a pooled quantitative analysis was not possible due to limited data, making it one of the limitations of our analysis.
IFX is more commonly used for IMC, likely due to several factors. As the first Food and Drug Administration (FDA)-approved SIT for inflammatory bowel disease (IBD), it was also the preferred biologic agent for managing steroid-refractory IMC due to physicians’ familiarity and comfort with its use [18]. As a relatively recent therapeutic option for IBD, VDZ has been less extensively studied in IMC compared to older agents, resulting in its limited use as first-line SIT and frequent insurance coverage restrictions. The above-mentioned results of our analysis back up the strategy proposed by Zou et al (2021), which is about employing rapid-induction IFX plus maintenance VDZ, as this approach would leverage the quick onset of action of IFX, followed by VDZ’s ability to maintain remission due to lower rates of recurrence of colitis along with a favorable safety profile [18].
Our analysis aligns with the findings of Zuberi et al, which demonstrated similar efficacy between IFX and VDZ in terms of remission of IMC, with response rates of 86.65% for IFX and 90.4% for VDZ [35]. However, unlike their analysis, which included studies with only one of these treatments, our analysis specifically focused on studies that involved direct comparisons between VDZ and IFX. Additionally, our analysis has demonstrated a pooled incidence of IMC recurrence and median duration of steroid exposure in patients receiving VDZ when compared to IFX, which were not reported in the prior meta-analysis.
The study has its limitations. Firstly, it is a meta-analysis of observational studies only, which might have influenced the effect size. Although the overall pooled OR for remission did show a favorable profile for VDZ over IFX, it was statistically insignificant due to the small sample size. However, the heterogeneity of the included studies was low for most of the parameters. So, the evidence is comprehensive and trustworthy, providing favorable support for the superiority of VDZ over IFX as a maintenance treatment in the treatment of IMC. A clinical trial at M.D. Anderson Cancer Center with trial ID NCT044007247 is the first ongoing randomized controlled trial (RCT) to evaluate the efficacy of VDZ over IFX, with preliminary results, but more prospective trials with larger sample sizes are required for more robust conclusions. Besides VDZ and IFX, previous studies have demonstrated that ustekinumab achieved remission in 68.4% of patients and calcineurin inhibitors in 72.7% of those with steroid-refractory IMC unresponsive to IFX or VDZ [36, 37]. Other biologic agents, such as mycophenolate, have also been utilized in the management of steroid-refractory IMC [38].
| Supplementary Material | ▴Top |
Suppl 1. Search strings (detailed search strategy with yielded results from all seven databases).
Acknowledgments
We appreciate the support of the Research Department at Rutgers Health/Community Medical Center.
Financial Disclosure
The authors have no relevant financial interests to disclose.
Conflict of Interest
The authors have no conflict of interest to declare that is relevant to the content of this article.
Informed Consent
Informed consent was not required for the study as it was a meta-analysis.
Author Contributions
SS, HS, and SC were involved in the conceptualization of the study. SS and HS performed the data collection and data synthesis. SS and HS, with the assistance of GR and CV, performed data analysis with statistics. SC carefully supervised manuscript preparation and writing. KA and VV thoroughly scrutinized the manuscript writing and edited it as required. MK and TB assisted with the overall editing and designing of tables and forest plots.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ICI: immune checkpoint inhibitor; CTLA-4: cytotoxic T-lymphocyte-associated antigen 4; PD-1: programmed death-1; irAEs: immune-related adverse effects; GI: gastrointestinal; IMC: immune-mediated colitis; IFX: infliximab; VDZ: vedolizumab; SIT: selective immunosuppressive therapy; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROSPERO: International Prospective Register of Systematic Review; PICOS: Patients; Interventions: ; Comparators: ; Outcomes and Studies: ; OR: odds ratio; CI: confidence interval; RC: retrospective cohort; MD: mean difference; TNF-A: tumor necrosis factor-alpha; IBD: inflammatory bowel disease; IV: intravenous
| References | ▴Top |
- Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131(1):58-67.
doi pubmed - Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027-1034.
doi pubmed - Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29-39.
doi pubmed - Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303-310.
doi pubmed - Marin-Acevedo JA, Harris DM, Burton MC. Immunotherapy-induced colitis: an emerging problem for the hospitalist. J Hosp Med. 2018;13(6):413-418.
doi pubmed - Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv119-iv142.
doi pubmed - Tandon P, Bourassa-Blanchette S, Bishay K, Parlow S, Laurie SA, McCurdy JD. The risk of diarrhea and colitis in patients with advanced melanoma undergoing immune checkpoint inhibitor therapy: a systematic review and meta-analysis. J Immunother. 2018;41(3):101-108.
doi pubmed - Menon T, Afzali A. Immune-mediated colitis. Curr Treat Options Gastroenterol. 2019;17(4):506-523.
doi pubmed - Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-1768.
doi pubmed - Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24(3):321-325.
doi pubmed - Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10(12):1437-1444.
doi pubmed - Johnson DH, Zobniw CM, Trinh VA, Ma J, Bassett RL, Jr., Abdel-Wahab N, Anderson J, et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer. 2018;6(1):103.
doi pubmed - Abu-Sbeih H, Ali FS, Alsaadi D, Jennings J, Luo W, Gong Z, Richards DM, et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study. J Immunother Cancer. 2018;6(1):142.
doi pubmed - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed - Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
doi pubmed - DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
doi pubmed - Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. Accessed April 4, 2025. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Zou F, Faleck D, Thomas A, Harris J, Satish D, Wang X, Charabaty A, et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two-center observational study. J Immunother Cancer. 2021;9(11):e003277.
doi pubmed - Abu-Sbeih H, Ali FS, Wang X, Mallepally N, Chen E, Altan M, Bresalier RS, et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2019;7(1):93.
doi pubmed - Badran YR, Zou F, Durbin SM, Dutra BE, Abu-Sbeih H, Thomas AS, Altan M, et al. Concurrent immune checkpoint inhibition and selective immunosuppressive therapy in patients with immune-related enterocolitis. J Immunother Cancer. 2023;11(6):e007195.
doi pubmed - Pizuorno Machado A, Shatila M, Glitza Oliva IC, Altan M, Siddiqui B, Zhou Y, Varatharajalu K, et al. Impact of selective immunosuppressive therapy on subsequent immune-related adverse events after immune checkpoint inhibitor-induced colitis treatment. Am J Clin Oncol. 2023;46(8):360-365.
doi pubmed - Joseph JA, Townsend MJ, Somappa AK, Sack JM, LeBoeuf NR, Sharon EM, Grover S. Infliximab versus vedolizumab in outpatient treatment of immune checkpoint inhibitor colitis. ACG 2024 Annual Scientific Meeting; 2024; Philadelphia, PA.
- Abu-Sbeih H, Ali FS, Luo W, Qiao W, Raju GS, Wang Y. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2018;6(1):95.
doi pubmed - Hashash JG, Francis FF, Farraye FA. Diagnosis and management of immune checkpoint inhibitor colitis. Gastroenterol Hepatol (N Y). 2021;17(8):358-366.
pubmed - Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, Okumura K. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med. 2002;195(2):161-169.
doi pubmed - Hsieh AH, Ferman M, Brown MP, Andrews JM. Vedolizumab: a novel treatment for ipilimumab-induced colitis. BMJ Case Rep. 2016;2016:bcr2016216641.
doi pubmed - Marsal J, Agace WW. Targeting T-cell migration in inflammatory bowel disease. J Intern Med. 2012;272(5):411-429.
doi pubmed - Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, Cohen J, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124(18):3706-3714.
doi pubmed - Bai X, Hu J, Betof Warner A, Quach HT, Cann CG, Zhang MZ, Si L, et al. Early use of high-dose glucocorticoid for the management of irAE is associated with poorer survival in patients with advanced melanoma treated with anti-PD-1 monotherapy. Clin Cancer Res. 2021;27(21):5993-6000.
doi pubmed - Lord JD, Hackman RC, Moklebust A, Thompson JA, Higano CS, Chielens D, Steinbach G, et al. Refractory colitis following anti-CTLA4 antibody therapy: analysis of mucosal FOXP3+ T cells. Dig Dis Sci. 2010;55(5):1396-1405.
doi pubmed - Kyi C, Hellmann MD, Wolchok JD, Chapman PB, Postow MA. Opportunistic infections in patients treated with immunotherapy for cancer. J Immunother Cancer. 2014;2:19.
doi pubmed - Arriola E, Wheater M, Krishnan R, Smart J, Foria V, Ottensmeier C. Immunosuppression for ipilimumab-related toxicity can cause pneumocystis pneumonia but spare antitumor immune control. Oncoimmunology. 2015;4(10):e1040218.
doi pubmed - Del Castillo M, Romero FA, Arguello E, Kyi C, Postow MA, Redelman-Sidi G. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis. 2016;63(11):1490-1493.
doi pubmed - Verheijden RJ, May AM, Blank CU, Aarts MJB, van den Berkmortel F, van den Eertwegh AJM, de Groot JWB, et al. Association of anti-TNF with decreased survival in steroid refractory ipilimumab and anti-PD1-treated patients in the dutch melanoma treatment registry. Clin Cancer Res. 2020;26(9):2268-2274.
doi pubmed - Zuberi S, Anderson K, Porth R, Taneja V, Feuerstein DJ. S314 Efficacy of infliximab and vedolizumab in immune checkpoint inhibitor colitis: a meta-analysis. American Journal of Gastroenterology. 2023;118(10S):S229-S231.
doi - Shirwaikar Thomas A, Lee SE, Shatila M, De Toni EN, Torok HP, Ben Khaled N, Powell N, et al. IL12/23 blockade for refractory immune-mediated colitis: 2-center experience. Am J Gastroenterol. 2023;118(9):1679-1683.
doi pubmed - Zhang E, Kiely C, Sandanayake N, Tattersall S. Calcineurin inhibitors in steroid and anti-TNF-alpha refractory immune checkpoint inhibitor colitis. JGH Open. 2021;5(5):558-562.
doi pubmed - Alcantar D, Al-Jaashaami L, Giron F. A case of immune checkpoint inhibitor refractory colitis treated with mycophenolate and high-dose steroids. Cureus. 2019;11(12):e6392.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.