| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 4, August 2025, pages 409-421
Upregulated E26 Transformation-Specific Variant Transcription Factor 7 in Oral Squamous Cell Carcinoma: Clinicopathological Correlations and Immune Regulatory Mechanisms
Xiang Zhi Yonga, b , Jian Di Lia, Yu Xing Tanga, Rong Quan Hec, Ping Lib, d, Ren Chuan Taob, e, Gang Chena, f
aDepartment of Pathology, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi 530021, China
bGuangxi Health Commission Key Laboratory of Prevention and Treatment for Oral Infectious Diseases, Nanning, Guangxi 530021, China
cDepartment of Oncology, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi 530021, China
dDepartment of Pathology, Hospital of Stomatology, Guangxi Medical University, Nanning, Guangxi 530021, China
eDepartment of Periodontal and Oral Medicine, College of Stomatology, Guangxi Medical University, Nanning, Guangxi 530021, China
fCorresponding Author: Gang Chen, Department of Pathology, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi 530021, China
Manuscript submitted May 3, 2025, accepted July 1, 2025, published online July 26, 2025
Short title: Upregulated ETV7 in OSCC
doi: https://doi.org/10.14740/wjon2606
| Abstract | ▴Top |
Background: E26 transformation-specific variant transcription factor 7 (ETV7) is implicated in various cancers, but its role in oral squamous cell carcinoma (OSCC) remains undefined. This study explores the clinicopathological significance and molecular mechanisms of ETV7 upregulation in OSCC.
Methods: ETV7 protein expression was assessed via immunohistochemistry (IHC) in 173 OSCC and 60 non-OSCC tissues. ETV7 mRNA levels were analyzed using bulk RNA sequencing and single-cell RNA sequencing, supplemented by immune infiltration, enrichment and cell communication analysis.
Results: IHC revealed significantly higher ETV7 protein expression in OSCC than in non-OSCC tissues (P < 0.001), correlating with advanced T (r = 0.380, P < 0.001) and N stages (r = 0.592, P < 0.001). High-throughput data confirmed ETV7 mRNA upregulation (standardized mean difference (SMD) = 0.35, 95% confidence interval (CI): 0.15 - 0.56; summary receiver operating characteristic (s receiver operating characteristic) area under the curve (AUC) = 0.78, 95% CI: 0.74 - 0.81), with levels decreasing twofold post-nivolumab treatment (P < 0.001). Enrichment analysis pinpointed the immune response-regulating signaling pathway as a key mechanism, supported by elevated immune cell infiltration (e.g., CD8+ T cells) in high-ETV7 samples. SLC15A4 and DAB2IP emerged as potentially overexpressed ETV7 targets. Cell communication analysis showed ETV7 enhancing myeloid cell interactions via the midkine (MK) pathway.
Conclusions: ETV7 upregulation drives OSCC progression, potentially through immune microenvironment modulation, positioning it as a candidate biomarker and therapeutic target. Its association with clinical stage and immunotherapy response underscores its prognostic relevance in OSCC management.
Keywords: ETV7; Oral squamous cell carcinoma; Immunohistochemistry; Immune microenvironment; RNA sequencing
| Introduction | ▴Top |
Oral cancer, predominantly affecting the oral cavity and lips, is the most prevalent malignancy among head and neck cancers [1]. In 2020, global estimates reported 377,713 new cases and 177,757 deaths [2]. Approximately 90% of these cases are oral squamous cell carcinomas (OSCC), arising from epithelial cells [3]. Despite prognostic advances over recent decades, OSCC remains an aggressive and unpredictable disease [4]. The immune microenvironment plays a pivotal role in OSCC progression and therapy response, yet its regulation remains poorly understood. Elucidating its molecular mechanisms is essential to improve understanding and therapeutic strategies.
E26 transformation-specific variant transcription factor 7 (ETV7), a member of the E26 transformation-specific (ETS) transcription factor family [5], is upregulated in several malignancies, including bladder cancer [6], hepatocellular carcinoma [7], and ovarian cancer [8]. ETV7 drives tumor progression through diverse pathways. For example, it interacts with mammalian target of rapamycin (mTOR) in the cytoplasm to form mTORC3, accelerating rhabdomyosarcoma onset and penetrance [9]. In breast cancer, ETV7 suppresses inflammation by downregulating TNFRSF1A [10]. However, its role in OSCC remains unexplored.
This study investigates ETV7’s potential contribution to OSCC using immunohistochemistry (IHC) and comprehensive high-throughput data analysis. By integrating these approaches, we aim to establish ETV7’s expression profile and functional significance, laying the groundwork for identifying novel mechanisms in OSCC management.
| Materials and Methods | ▴Top |
IHC
ETV7 protein expression was assessed using IHC on five tissue microarrays (TMAs) comprising 173 OSCC and 60 non-OSCC samples (Fanpu Biotech, Inc., Guilin, China; TMA IDs: TOC481, ORC1021, HNT961, HNT962, HNT1021). The study was approved by the Ethical Committee of College of Stomatology, Guangxi Medical University (Institutional Review Board (IRB):2023039) and was conducted in accordance with the ethical standards of the Helsinki Declaration. IHC was performed using an anti-ETV7 antibody (Abcam, ab229832) per the manufacturer’s protocol. Positive staining was scored based on area percentage: 0 (0-10%), 1 (11-25%), 2 (26-50%), 3 (51-75%), or 4 (76-100%), and staining intensity: 1 (weak), 2 (medium), or 3 (strong). The final ETV7 IHC score was calculated as the product of the positive cell percentage score and staining intensity score through blinded evaluation by two independent assessors [8].
Bulk mRNA sequencing (mRNA-seq) analysis
Microarray and RNA-seq datasets were retrieved from public databases including Gene Expression Omnibus (GEO), Sequence Read Archive (SRA), The Cancer Genome Atlas (TCGA), and ArrayExpress to evaluate ETV7 mRNA expression in OSCC. Inclusion criteria were: 1) human OSCC tissues; 2) available ETV7 expression data; and 3) ≥ three samples per experimental and control group. Batch effects were corrected, and ETV7 mRNA levels in OSCC versus control groups were visualized using boxplots and receiver operating characteristic (ROC) curves.
scRNA-seq data analysis
Single-cell RNA sequencing (scRNA-seq) data were sourced from GEO with inclusion criteria: 1) OSCC and normal tissue samples; 2) human-derived samples. Exclusion criteria included: 1) drug-treated or metastatic samples; 2) immune-cell-only datasets; and 3) spatial transcriptomics data. Datasets were merged using the merge function, and batch effects were adjusted with the Harmony package. High-quality cells were filtered (200 < nFeature_RNA < 2,500, mitochondrial gene content < 5%). Dimensionality reduction was performed using Uniform Manifold Approximation and Projection (UMAP), followed by clustering at a resolution of 0.2 and cell-type annotation [11].
Immuno-infiltration analysis
TCGA-OSCC log (TPM+1) sequencing data were analyzed. Tumor Immune Estimation Resource (TIMER) 2 and the xCell deconvolution algorithm assessed the relative abundance of immune and mesenchymal cells in OSCC. Immune cell levels were compared between high- and low-ETV7 mRNA expression groups, and Pearson correlation was calculated to evaluate ETV7’s association with immune infiltration.
Cell communication analysis
Malignant epithelial cells were stratified into high- and low-ETV7 expression groups. The CellChat package [12] was used to analyze interactions between the high-ETV7 group and other cell types, focusing on ligand-receptor pair communication strength and functional implications.
Enrichment analysis
OSCC-overexpressed genes (standardized mean difference (SMD) > 0, P < 0.05) and ETV7 coexpressed genes (Pearson r ≥ 0.30, P < 0.05, significant in ≥ three datasets) were identified from batch-corrected data. ETV7 regulatory candidates were screened via CistromeDB (score ≥ 0.50). Overlapping genes among OSCC-overexpressed, ETV7 coexpressed, and ETV7-regulated sets were subjected to Gene Ontology (GO) and Reactome enrichment analysis using clusterProfiler.
Validation of ETV7 overexpression and gene dependency in OSCC
Gene dependency was assessed using the Cancer Dependency Map. The “CRISPR (DepMap Public 24Q4 + Score Chronos)” dataset provided gene effect scores for OSCC cell lines post-ETV7 knockout. The Chronos algorithm evaluated cell viability impacts, with negative scores indicating growth inhibition following ETV7 knockout.
Statistical analysis
ETV7 protein expression differences between OSCC and control groups were analyzed using independent-samples t-tests, while mRNA levels were assessed with the Wilcoxon test. Spearman correlation examined associations between ETV7 protein levels and clinical parameters. Ordinal logistic regression was employed to evaluate the impact of ETV7 protein expression levels, gender, and TNM staging on clinical stage outcomes. ROC curves and area under the curve (AUC) values evaluated ETV7 protein’s discriminatory capacity. ETV7 mRNA expression and diagnostic ability were summarized using SMD forest plots and summary receiver operating characteristic (sROC) curves. Publication bias was assessed with Egger’s and Begg’s tests. Statistical significance was set at P < 0.05.
| Results | ▴Top |
The protein overexpression and clinical relevance of ETV7 in OSCC tissues
ETV7 protein expression varied markedly between non-OSCC and OSCC tissues (Fig. 1a, b). In non-OSCC tissues, ETV7 expression was generally low, with only occasional positive staining in epithelial cells. Some samples exhibited no cytoplasmic ETV7 signal, showing only faint staining in intercellular squamous epithelial bridges (Fig. 1a). In contrast, OSCC tissues displayed strong, uniform ETV7 expression, with positive signals localized to the cytoplasm (Fig. 1b). Significantly higher ETV7 protein levels were confirmed in OSCC compared to non-OSCC tissues (P < 0.001) (Fig. 1c). ROC analysis further validated this overexpression, yielding an AUC near 1 (Fig. 1d), demonstrating ETV7’s ability to distinguish OSCC from non-OSCC tissues.
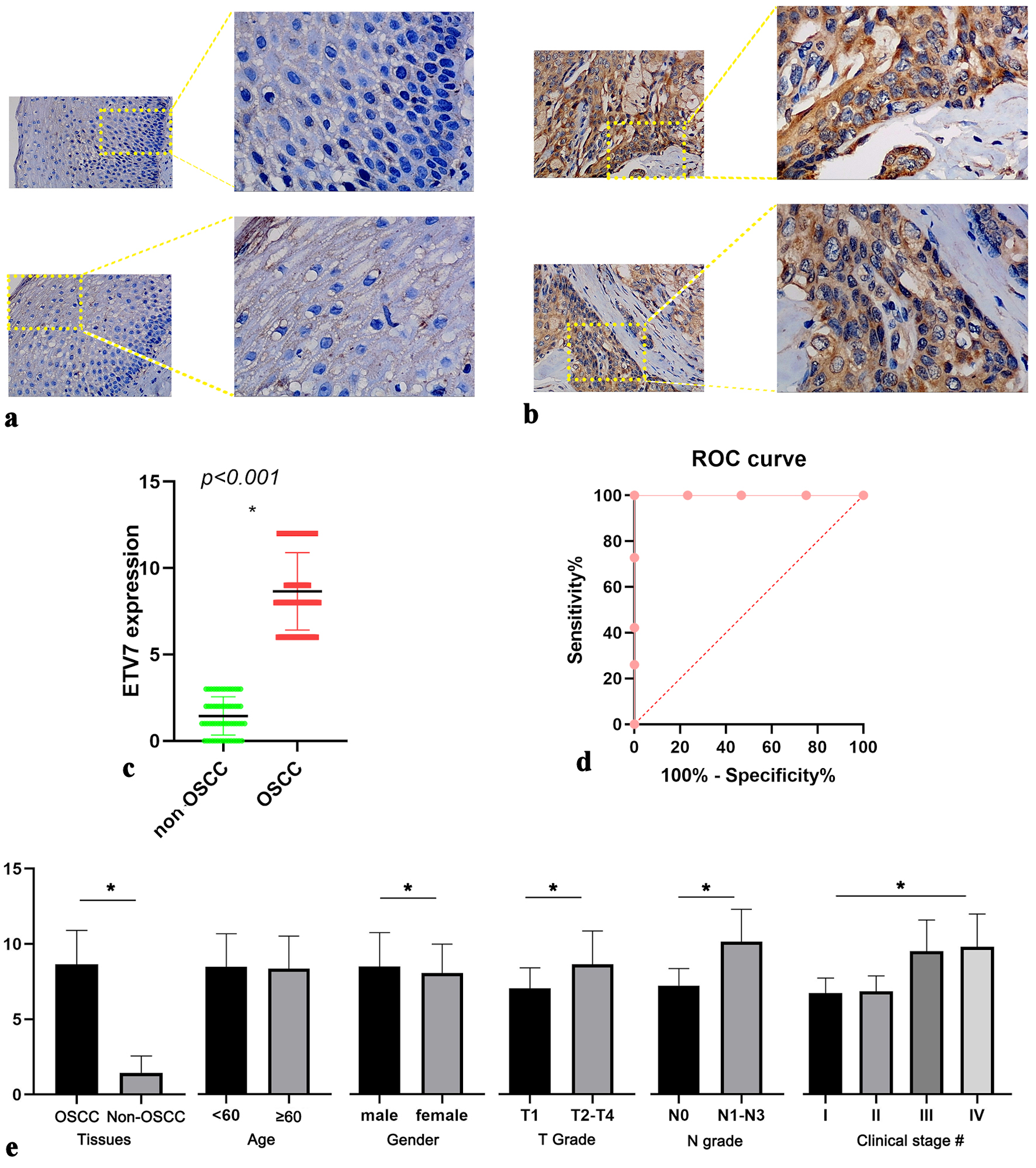 Click for large image | Figure 1. IHC staining of ETV7 expression in OSCC. (a) Expression of ETV7 in non-OSCC tissues. (b) Expression of ETV7 in OSCC tissues. (c) Different expression of ETV7 protein between non-OSCC and OSCC tissues. (d) ROC analysis of ETV7 protein expression in OSCC tissues. (e) Relationship between ETV7 protein level and clinicopathological parameters of OSCC patients based on the IHC. *P < 0.05. #Clinical stage: I vs. III, I vs. IV, II vs. III, II vs. IV, P < 0.001. Early stage (I and II) vs. advanced stage (III and IV) P < 0.001. IHC: immunohistochemistry; ETV7: E26 transformation-specific variant transcription factor 7; OSCC: oral squamous cell carcinoma; ROC: receiver operating characteristic. |
The relationship between ETV7 protein expression and OSCC clinical parameters was also examined. Elevated ETV7 levels were observed in male patients and those with T2-4, N1-3, and advanced clinical stages compared to their counterparts (Fig. 1e). Spearman correlation analysis revealed a strong positive association between ETV7 expression and clinical stage (r = 0.618, P < 0.001), with notable correlations to T stage (r = 0.380, P < 0.001) and N stage (r = 0.592, P < 0.001). But multiple linear regression analysis revealed that ETV7 expression score was not a significant independent effect on clinical stage (P = 0.952).
Upregulation of ETV7 mRNA in OSCC
High-throughput data from 12 platforms were analyzed (Table 1), confirming ETV7 mRNA upregulation in OSCC (SMD = 0.35, 95% CI: 0.15 - 0.56; sROC AUC = 0.78, 95% CI: 0.74 - 0.81) (Fig. 2). These findings suggest ETV7 may contribute to the biological and clinical progression of OSCC.
 Click to view | Table 1. Information of Included High Throughput Data |
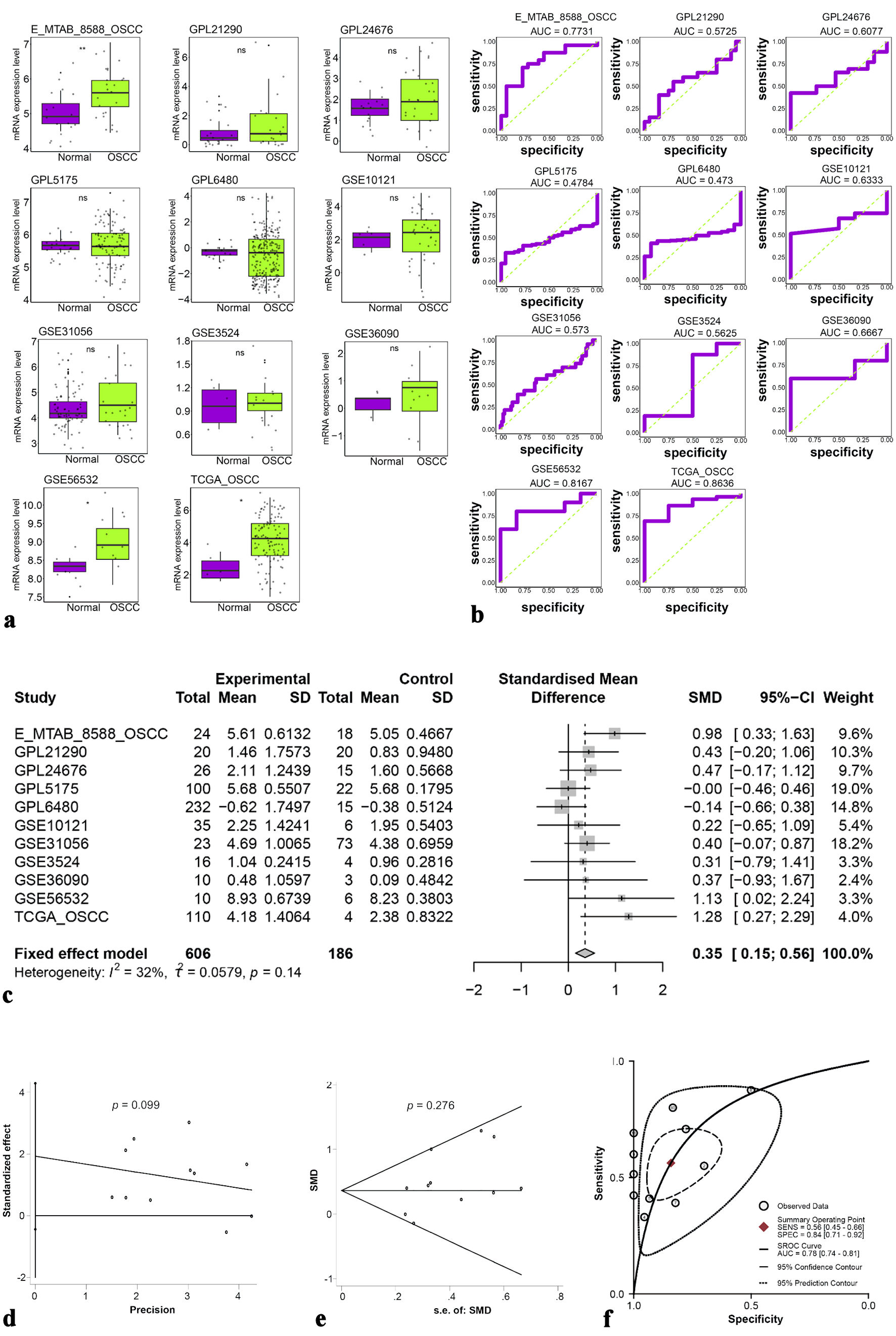 Click for large image | Figure 2. (a) Different expressions of ETV7 mRNA. (b) ROC analysis of ETV7 mRNA expression in each dataset. (c) Forest plot of standardized mean differences of ETV7 mRNA. (d, e) Publication bias test of included datasets. (f) sROC curve of ETV7 mRNA. ETV7: E26 transformation-specific variant transcription factor 7; ROC: receiver operating characteristic; sROC: summary receiver operating characteristic; SD: standard deviation; SMD: standardized mean difference; CI: confidence interval. |
Additionally, four scRNA-seq datasets from the GEO database (GSE164241, GSE172577, GSE195655, GSE195832) were integrated, normalized, and corrected for batch effects (Fig. 3a). Cell-type annotation delineated the distribution of subpopulations within the OSCC microenvironment (Fig. 3b). ETV7 mRNA expression was significantly elevated in malignant epithelial cells, T cells, B cells, neutrophils, myeloid cells, endothelial cells, and stromal cells (Fig. 3c). Notably, ETV7 expression decreased twofold following treatment with the programmed cell death protein 1 (PD-1) inhibitor nivolumab (P < 0.001) (Fig. 3d).
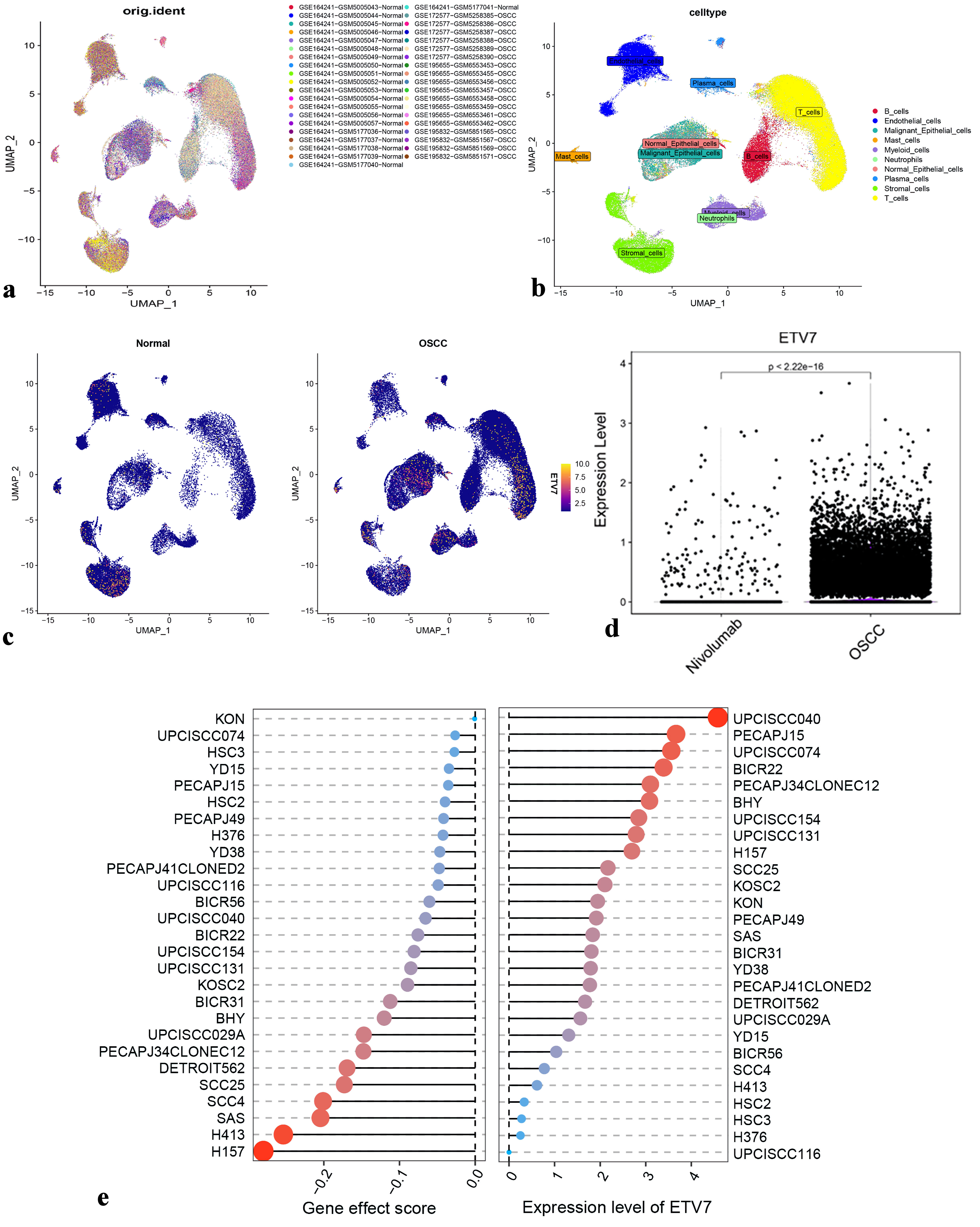 Click for large image | Figure 3. Expression of ETV7 in OSCC different subpopulations. (a) Cluster analysis for scRNA-seq data. (b) Cell annotation for clusters. (c) Density plot of ETV7 expression distribution in each cluster. (c) Changes of OSCC cell subsets after nivolumab treatment. (e) ETV7 expression vs. gene effect in head and neck cancer cell lines. ETV7: E26 transformation-specific variant transcription factor 7; OSCC: oral squamous cell carcinoma; scRNA-seq: single-cell RNA sequencing. |
Validation of ETV7 expression and gene dependency in OSCC
ETV7 expression varied across OSCC cell lines. Clustered regularly interspaced short palindromic repeats (CRISPR)-based screening revealed that ETV7 knockout inhibited the growth of 27 OSCC cell lines, as indicated by negative gene effect scores < 0 (Fig. 3e).
Potential mechanism of ETV7 in OSCC
Given ETV7’s role as a transcription factor and its elevated expression in OSCC, we intersected OSCC-overexpressed genes, ETV7 positively coexpressed genes, and potential ETV7 target genes for further analysis (Fig. 4a). Enrichment analysis of this intersection, detailed in Figure 4b, highlighted significant enrichment in biological processes and Reactome pathways, including immune response-regulating signaling, interferon signaling, and interleukin signaling. Based on the observed reduction in ETV7 expression following immunotherapy, we focused on the immune response-regulating signaling pathway, constructing a protein-protein interaction (PPI) network (Fig. 4c). This analysis revealed potential positive regulatory relationships between ETV7 and SLC15A4 and DAB2IP within this pathway (Fig. 4d, e). Subsequent investigation confirmed that SLC15A4 and DAB2IP, both highly expressed in OSCC, are likely ETV7 targets (Fig. 4f, g).
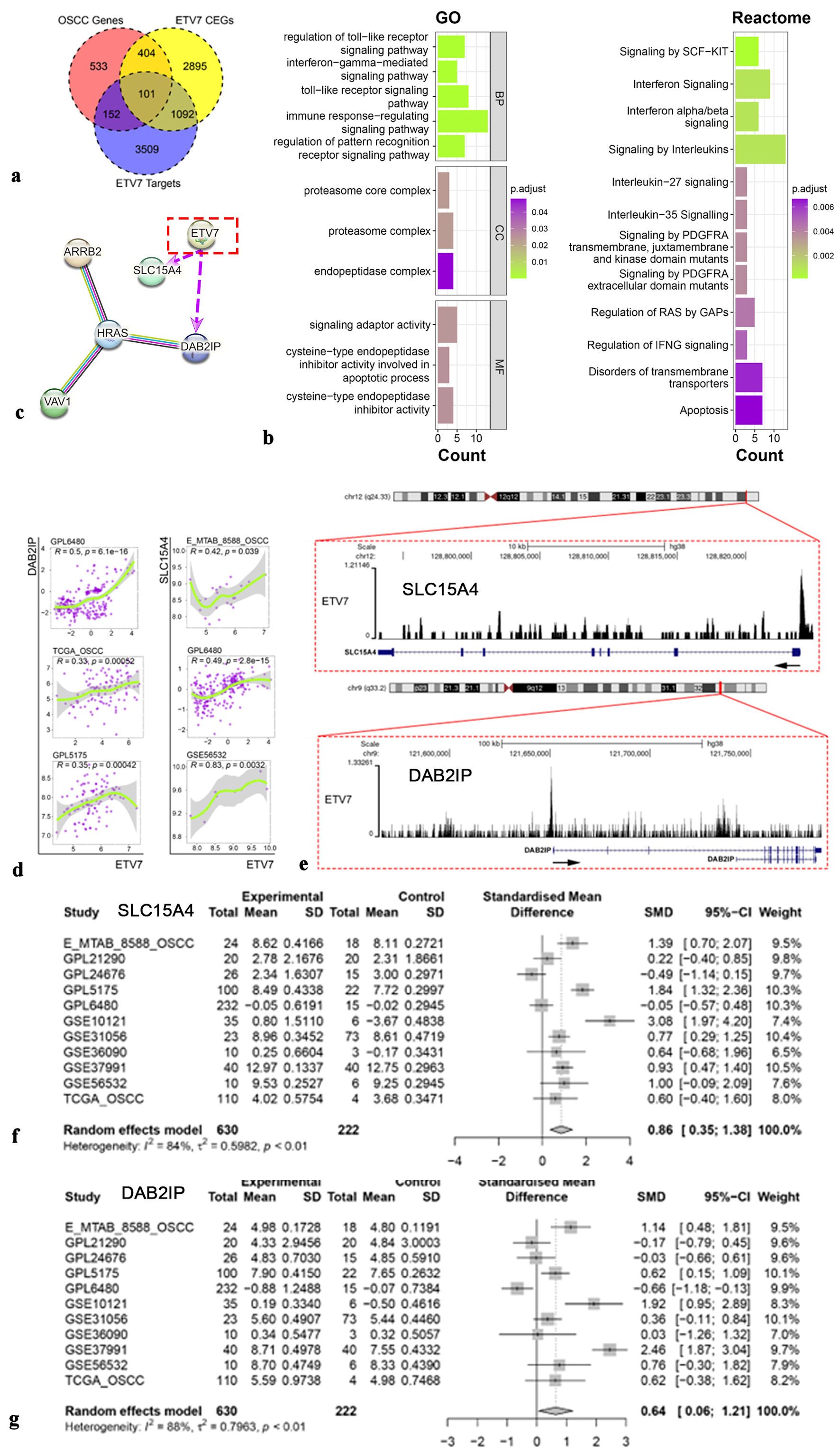 Click for large image | Figure 4. Potential mechanism of ETV7 in OSCC. (a) The intersection of OSCC overexpressed genes, ETV7 coexpressed genes, and ETV7 targets genes. (b) Enrichment analysis for ETV7. (c) Protein-protein interactive network for ETV7 (purple arrows indicate potential positive transcriptional regulatory relationships for ETV7). (d) The relationship between ETV7 and targeted genes SLC15A4 and DAB2IP. (e) SLC15A4 and DAB2IP were potential targets of ETV7. (f) Different expression of SLC15A4 mRNA in OSCC. (g) Different expression of DAB2IP mRNA in OSCC. ETV7: E26 transformation-specific variant transcription factor 7; OSCC: oral squamous cell carcinoma; SD: standard deviation; SMD: standardized mean difference; CI: confidence interval. |
scRNA-seq indicated ETV7 expression in immune cells, prompting an investigation into its relationship with immune infiltration. Analysis revealed differential immune cell abundance corresponding to ETV7 expression levels (Fig. 5a). Higher ETV7 expression was associated with increased immune infiltration, showing a positive correlation overall. Specifically, ETV7 expression positively correlated with infiltration of CD4+ T cells, CD8+ T cells, dendritic cells (DCs), M1 macrophages, and memory B cells (Fig. 5b). Malignant epithelial cells in the OSCC group were stratified into high-ETV7 (clusters 0, 1, 7, 8) and low-ETV7 (clusters 2, 3, 4, 5, 6, 9) expression groups based on ETV7 mRNA levels (Fig. 6a). Cell communication analysis demonstrated significantly enhanced interactions between the high-ETV7 group and myeloid cells (Fig. 6b). Additionally, the high-ETV7 group exhibited a strong association with the midkine (MK) signaling pathway (Fig. 6c), where it served as a key “influencer”, receiving MK pathway signals (Fig. 6d, e). Ligand-receptor interaction analysis further revealed significant enrichment of MK pathway-related pairs in the high-ETV7 group, including midkine (MDK)-nucleolin (NCL), MDK-(integrin subunit alpha 6 (ITGA6) + integrin subunit beta 1 (ITGB1), and MDK-syndecan-4 (SDC4) (Fig. 6f).
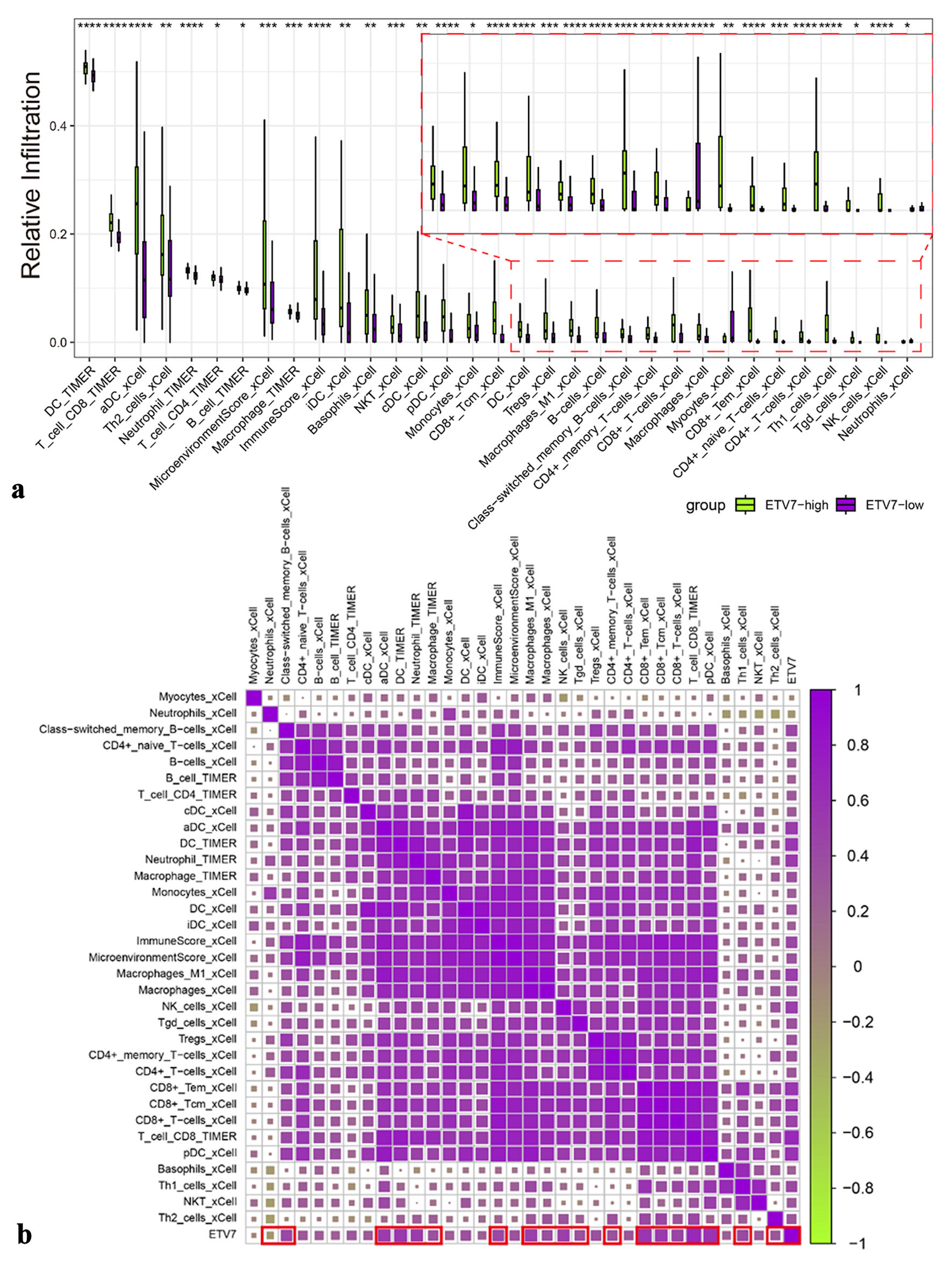 Click for large image | Figure 5. The relationship between immune infiltration and ETV7 expression. (a) Immune infiltration with different expressions of ETV7. (b) Correlation between ETV7 and immune infiltration. ETV7: E26 transformation-specific variant transcription factor 7. |
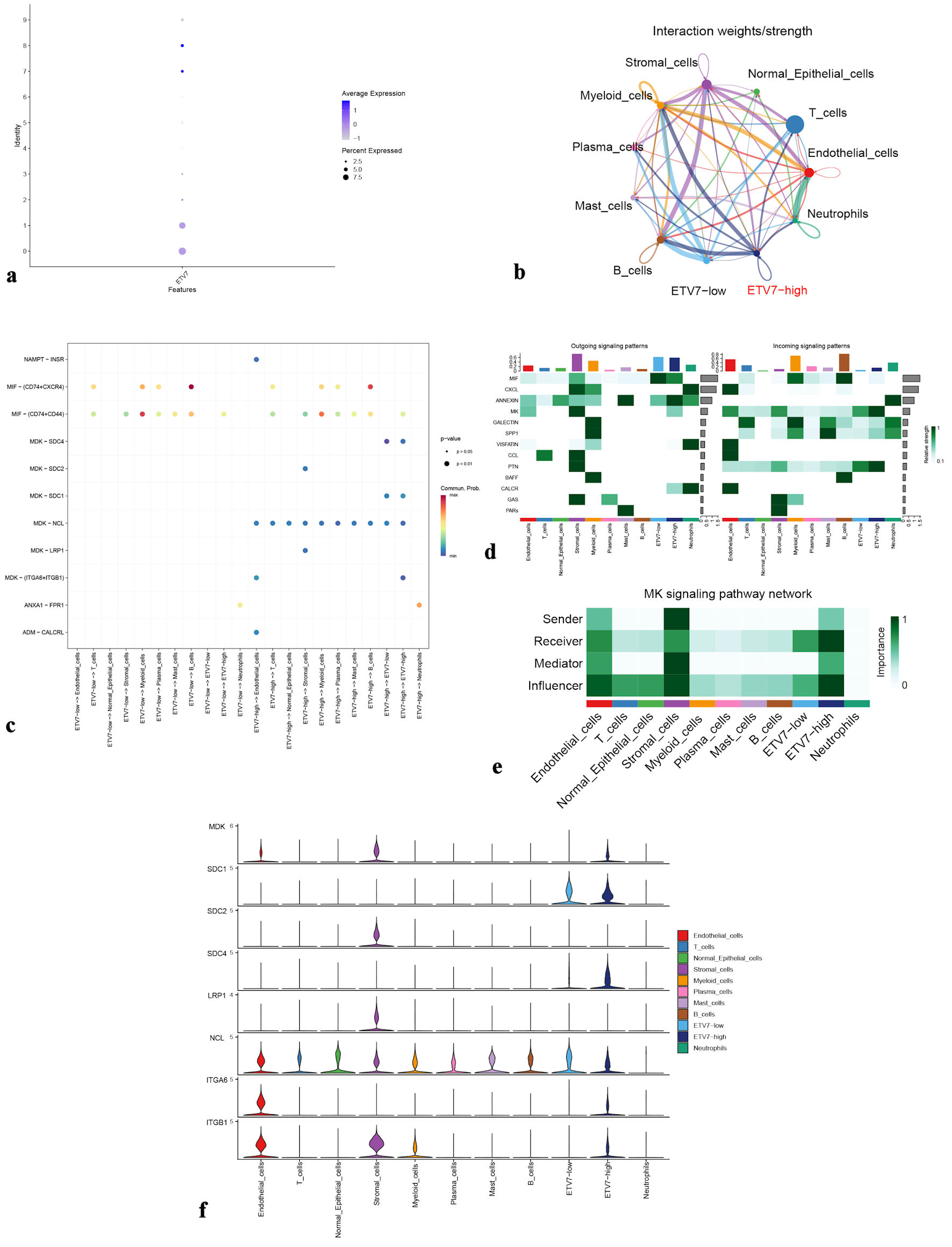 Click for large image | Figure 6. (a) Dot plot showing ETV7 expression in epithelial cell clusters from the OSCC group. (b) Cellular interaction network among different cell types. (c) Ligand-receptor signaling patterns between ETV7 high-expression and low-expression groups. (d) Heatmap of output and input signaling patterns. (e) Midkine (MK) signaling pathway network. (f) Violin plots illustrate expression levels of MK pathway-associated genes. ETV7: E26 transformation-specific variant transcription factor 7; OSCC: oral squamous cell carcinoma. |
| Discussion | ▴Top |
This study confirmed elevated ETV7 expression in OSCC at both protein and mRNA levels, exploring its mechanisms through functional enrichment, immune infiltration, and cell communication analyses.
ETV7, recognized as an oncoprotein in various solid tumors [6] and hematological malignancies [13], remains poorly characterized in OSCC. We initiated our study by assessing ETV7 protein expression via IHC, followed by mRNA analysis using publicly available high-throughput datasets. Our results consistently showed elevated ETV7 expression in OSCC tissues compared to non-OSCC controls at both protein and mRNA levels. Clinical analysis further revealed a positive correlation between ETV7 overexpression and OSCC progression, particularly with advanced clinical stages. scRNA-seq demonstrated a significant reduction in ETV7 expression following immunotherapy, coinciding with decreased tumor cell populations, while CRISPR-mediated ETV7 knockout inhibited growth in 27 OSCC cell lines. These findings collectively underscore ETV7’s critical role in driving OSCC pathogenesis.
To further explore ETV7’s role in OSCC, we employed bioinformatics approaches to investigate its underlying mechanisms. Gene enrichment analysis revealed that ETV7 is primarily involved in immune response-regulating signaling, interferon signaling, and interleukin signaling pathways. Previous studies have implicated ETV7 in immune modulation, notably by promoting CD8+ T-cell infiltration in melanoma through regulation of chemokines (e.g., CC motif chemokine ligand 5 (CCL5) and CXC motif chemokine ligand 10 (CXCL10)) [14] and enhancing antigen processing and presentation via major histocompatibility complex (MHC) class I molecules in urothelial carcinoma [15]. Moreover, mouse model studies have demonstrated that ETV7 overexpression binds to the promoter regions of genes such as TCF7, CTLA4, and TOX, driving CD8+ T-cell differentiation from a memory to an exhausted state, thereby impairing their anti-tumor efficacy. Consequently, increased CD8+ T-cell infiltration is observed in tumors [16]. Consistent with these findings, our study revealed elevated CD8+ T-cell infiltration in the high-ETV7 expression group compared to the low-expression group. Thus, in chimeric antigen receptor (CAR)-T cell therapy, knocking down ETV7 expression could enhance the anti-tumor efficacy of CAR-T cells and reduce the proportion of exhausted cells, offering a promising novel therapeutic strategy for OSCC [16]. Additionally, as an interferon-stimulated gene (ISG), ETV7 regulates interferon responses [17] and has been shown to suppress interferon-responsive genes, thereby influencing breast cancer stem cell plasticity and resistance to chemotherapy and radiotherapy [18]. Modulating ETV7 expression or activity may improve therapeutic outcomes in breast cancer, reduce drug resistance, and lower the risk of recurrence [19].
Focusing on the immune response-regulating signaling pathway, we identified SLC15A4 and DAB2IP as potential ETV7 target genes. SLC15A4, a member of the solute carrier (SLC) family of membrane transporters [20], includes four mammalian isoforms: SLC15A1 (PEPT1), SLC15A2 (PEPT2), SLC15A3 (PHT2), and SLC15A4 (PHT1). It facilitates histidine transport across cell membranes [21] and is implicated in immune disorders such as systemic lupus erythematosus [22] and ulcerative colitis [23]. SLC15A4 regulates the Toll-like receptor (TLR)-triggered interferon regulatory factor 7 (IRF7)-type I interferon (IFN-I) axis, enhances inflammasome activity via mTORC1 signaling, and contributes to IFN-I production through the mTOR pathway [24, 25], suggesting a role in modulating the tumor immune microenvironment. Elevated SLC15A4 expression has been proposed as a prognostic biomarker in lung adenocarcinoma [26] and observed in colorectal [27] and prostate cancers [28], consistent with our findings of high SLC15A4 expression in OSCC. Besides, DAB2IP modulates the interactions between tumor cells and immune cells, thereby influencing the immune status of the tumor microenvironment (TME). Studies have shown that downregulation of DAB2IP may enhance the activation of the nuclear factor kappa B (NF-κB) signaling pathway, promoting the expression of pro-inflammatory cytokines (e.g., tumor necrosis factor alpha (TNF-α)), which subsequently shapes a pro-tumor immune microenvironment [29]. The expression level of DAB2IP is closely associated with immune cell infiltration in tumors. For example, in renal cell carcinoma, reduced DAB2IP expression correlates with decreased infiltration of CD8+ T cells and B cells, which may contribute to exacerbated immunosuppression and poor prognosis [30]. Additionally, as an IFN-responsive gene, DAB2IP plays a regulatory role in the IFN signaling pathway. Studies reveal that DAB2IP suppresses the overactivation of IFN-responsive genes, a mechanism implicated in modulating the plasticity of cancer stem cells and therapy resistance in breast cancer [31]. SLC15A4 and DAB2IP, as potential target genes of ETV7, play critical roles in the TME by regulating immune-related signaling pathways. These findings suggest that they may serve as key molecular hubs connecting ETV7 to the remodeling of the immune microenvironment in OSCC.
Additionally, cell communication analysis revealed that ETV7 high-expression cells likely interact closely with myeloid cells via macrophage migration inhibitory factor (MIF)-(CD74 + CXC chemokine receptor type 4 (CXCR4)) and MIF-(CD74 + CD44) ligand-receptor pairs. Myeloid cells are critical indicators of OSCC prognosis and immune infiltration. Increased myeloid cell presence, driven by cancer-associated inflammation, promotes the secretion of pro-inflammatory cytokines, enhancing cancer cell proliferation and metastasis [32]. Additionally, the ETV7 high-expression group showed significant enrichment in MK pathway-related ligand-receptor pairs, including MDK-NCL and MDK-SDC4. The MK pathway, previously identified in colorectal cancer, pancreatic ductal adenocarcinoma, and breast cancer, facilitates signaling from malignant cells to the TME interface. In this pathway, malignant cells secrete MDK, engaging receptors NCL and SDC4 to foster an immunosuppressive and angiogenic TME that drives tumor growth [33]. These findings suggest that in OSCC, ETV7 high-expression cells may modulate the tumor immune environment and promote metastasis through MDK-mediated signaling. Research has revealed that MDK can be recognized by the T-cell receptor of CD8+ T cells, thereby triggering an immune response. MDK-specific cytotoxic T lymphocytes are capable of lysing tumor cells [34]. However, MDK also contributes to the formation of immunosuppressive TME, which impairs the activity and function of CD8+ T cells, leading to their exhaustion and reducing their ability to effectively target tumor cells [35]. Given MDK’s critical role in modulating the TME and influencing CD8+ T cell function, inhibitors of the MDK signaling pathway could be combined with immune checkpoint inhibitors or other immunotherapeutic agents to enhance the efficacy of immunotherapy [36]. This study provides valuable insights into ETV7’s role in OSCC; however, several limitations should be acknowledged. First, the reliance on retrospective TMAs and publicly available datasets may limit the generalizability of findings across diverse OSCC populations. Second, while bioinformatics analyses identified SLC15A4 and DAB2IP as potential ETV7 targets, direct experimental validation (e.g., ChIP-seq or luciferase assays) is lacking to confirm these regulatory relationships. Third, the study’s focus on immune-related pathways, such as the MK-MDK signaling axis, leaves other potential mechanisms (e.g., metabolic or proliferative pathways) underexplored. Finally, the functional impact of ETV7 knockout was assessed in OSCC cell lines, but in vivo models are needed to better recapitulate the TME and clinical context. These gaps highlight the need for further studies to strengthen the translational potential of our findings.
Conclusions
In conclusion, our study demonstrates ETV7 overexpression in OSCC and underscores its critical role in disease progression, likely mediated by its influence on the immune microenvironment. The precise mechanisms underlying these findings warrant further experimental investigation.
Acknowledgments
This research was supported by the Guangxi Young Elite Scientist Sponsorship Program (GXYESS2025217) and First-Class Discipline Innovation-Driven Talent Program of Guangxi Medical University.
Financial Disclosure
This research was supported by grants from the China Postdoctoral Science Foundation (2025MD774065); the National Science Foundation of China (82460191); the Guangxi Higher Education Undergraduate Teaching Reform Project (2022JGA146); and the Guangxi Educational Science Planning Key Project (2022ZJY2791).
Conflict of Interest
The authors declare that there is no conflict of interest. All authors read and approved the final manuscript. There is no conflict of interest with any companies regarding this manuscript.
Informed Consent
The requirement for informed consent was waived by the IRB.
Author Contributions
Xiang Zhi Yong participated in designing the study, analyzing the data and drafting of article; Jian Di Li, Yu Xing Tang and Rong Quan He participated in collecting the high throughput data and analyzing the data; Ping Li participated in collecting tissue and conducting IHC; Ren Chuan Tao participated in designing the study, drafting of article and critical revision; Gang Chen participated in designing the study, critical revision and financial support. All authors reviewed the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
ETV7, E26 transformation-specific variant transcription factor 7; OSCC, oral squamous cell carcinoma; IHC, immunohistochemistry; GEO, Gene Expression Omnibus; SRA, Sequence Read Archive; TCGA, The Cancer Genome Atlas; ROC, receiver operating characteristic; scRNA-seq, single-cell RNA sequencing
| References | ▴Top |
- Global Health Estimates 2020: deaths by cause, age, sex, by country and by region, 2000-2019. [who.int/data/gho/data/themes/mortality-andglobal-health-estimates/gheleadingcauses-of-death].
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Miranda-Filho A, Bray F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020;102:104551.
doi pubmed - Jha A, Nath N, Kumari A, Kumari N, Panda AK, Mishra R. Polymorphisms and haplotypes of TLR-4/9 associated with bacterial infection, gingival inflammation/recession and oral cancer. Pathol Res Pract. 2023;241:154284.
doi pubmed - Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2(11):827-837.
doi pubmed - Li H, Zhang Y, Zheng S. Comprehensive analysis identified ETV7 as a potential prognostic biomarker in bladder cancer. Biomed Res Int. 2021;2021:8530186.
doi pubmed - Matos JM, Witzmann FA, Cummings OW, Schmidt CM. A pilot study of proteomic profiles of human hepatocellular carcinoma in the United States. J Surg Res. 2009;155(2):237-243.
doi pubmed - Huo X, Sun H, Liu S, Liang B, Bai H, Wang S, Li S. Identification of a prognostic signature for ovarian cancer based on the microenvironment genes. Front Genet. 2021;12:680413.
doi pubmed - Harwood FC, Klein Geltink RI, O'Hara BP, Cardone M, Janke L, Finkelstein D, Entin I, et al. ETV7 is an essential component of a rapamycin-insensitive mTOR complex in cancer. Sci Adv. 2018;4(9):eaar3938.
doi pubmed - Meskyte EM, Pezze L, Bartolomei L, Forcato M, Bocci IA, Bertalot G, Barbareschi M, et al. ETV7 reduces inflammatory responses in breast cancer cells by repressing the TNFR1/NF-kappaB axis. Cell Death Dis. 2023;14(4):263.
doi pubmed - Hu S, Lu H, Xie W, Wang D, Shan Z, Xing X, Wang XM, et al. TDO2+ myofibroblasts mediate immune suppression in malignant transformation of squamous cell carcinoma. J Clin Invest. 2022;132(19):e157649.
doi pubmed - Xiong K, Fang Y, Qiu B, Chen C, Huang N, Liang F, Huang C, et al. Investigation of cellular communication and signaling pathways in tumor microenvironment for high TP53-expressing osteosarcoma cells through single-cell RNA sequencing. Med Oncol. 2024;41(5):93.
doi pubmed - Rasighaemi P, Ward AC. ETV6 and ETV7: Siblings in hematopoiesis and its disruption in disease. Crit Rev Oncol Hematol. 2017;116:106-115.
doi pubmed - Qu H, Zhao H, Zhang X, Liu Y, Li F, Sun L, Song Z. Integrated analysis of the ETS family in melanoma reveals a regulatory role of ETV7 in the immune microenvironment. Front Immunol. 2020;11:612784.
doi pubmed - Wang Y, Yan K, Lin J, Liu Y, Wang J, Li X, Li X, et al. CD8+ T cell co-expressed genes correlate with clinical phenotype and microenvironments of urothelial cancer. Front Oncol. 2020;10:553399.
doi pubmed - Cheng J, Xiao Y, Peng T, Zhang Z, Qin Y, Wang Y, Shi J, et al. ETV7 limits the antiviral and antitumor efficacy of CD8(+) T cells by diverting their fate toward exhaustion. Nat Cancer. 2025;6(2):338-356.
doi pubmed - Froggatt HM, Harding AT, Chaparian RR, Heaton NS. ETV7 limits antiviral gene expression and control of influenza viruses. Sci Signal. 2021;14(691):eabe1194.
doi pubmed - Pezze L, Meskyte EM, Forcato M, Pontalti S, Badowska KA, Rizzotto D, Skvortsova, II, et al. ETV7 regulates breast cancer stem-like cell features by repressing IFN-response genes. Cell Death Dis. 2021;12(8):742.
doi pubmed - Khan ZS, Hussain F. Curbing breast cancer by altering V-ATPase action on F-Actin, heterochromatin, ETV7 and mTORC2 signaling. Cell Physiol Biochem. 2024;58(3):250-272.
doi pubmed - Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004;447(5):465-468.
doi pubmed - Song F, Yi Y, Li C, Hu Y, Wang J, Smith DE, Jiang H. Regulation and biological role of the peptide/histidine transporter SLC15A3 in Toll-like receptor-mediated inflammatory responses in macrophage. Cell Death Dis. 2018;9(7):770.
doi pubmed - Li S, Wu Q, Jiang Z, Wu Y, Li Y, Ni B, Xiao J, et al. miR-31-5p regulates type i interferon by targeting SLC15A4 in plasmacytoid dendritic cells of systemic lupus erythematosus. J Inflamm Res. 2022;15:6607-6616.
doi pubmed - Mazzei A, Serino G, Romano A, Piccinno E, Scalavino V, Valentini AM, Armentano R, et al. Identification of SLC15A4/PHT1 gene products upregulation marking the intestinal epithelial monolayer of ulcerative colitis patients. Int J Mol Sci. 2022;23(21):13170.
doi pubmed - Kobayashi T, Shimabukuro-Demoto S, Yoshida-Sugitani R, Furuyama-Tanaka K, Karyu H, Sugiura Y, Shimizu Y, et al. The histidine transporter SLC15A4 coordinates mTOR-dependent inflammatory responses and pathogenic antibody production. Immunity. 2014;41(3):375-388.
doi pubmed - Lopez-Haber C, Netting DJ, Hutchins Z, Ma X, Hamilton KE, Mantegazza AR. The phagosomal solute transporter SLC15A4 promotes inflammasome activity via mTORC1 signaling and autophagy restraint in dendritic cells. EMBO J. 2022;41(20):e111161.
doi pubmed - Huang H, Wang J, Chen S, He H, Shang Y, Guo X, Lou G, et al. SLC15A4 Serves as a Novel Prognostic Biomarker and Target for Lung Adenocarcinoma. Front Genet. 2021;12:666607.
doi pubmed - Lee YS, Kim BH, Kim BC, Shin A, Kim JS, Hong SH, Hwang JA, et al. SLC15A2 genomic variation is associated with the extraordinary response of sorafenib treatment: whole-genome analysis in patients with hepatocellular carcinoma. Oncotarget. 2015;6(18):16449-16460.
doi pubmed - Tai W, Chen Z, Cheng K. Expression profile and functional activity of peptide transporters in prostate cancer cells. Mol Pharm. 2013;10(2):477-487.
doi pubmed - Bellazzo A, Di Minin G, Valentino E, Sicari D, Torre D, Marchionni L, Serpi F, et al. Cell-autonomous and cell non-autonomous downregulation of tumor suppressor DAB2IP by microRNA-149-3p promotes aggressiveness of cancer cells. Cell Death Differ. 2018;25(7):1224-1238.
doi pubmed - Cao H, Zhang J, Wang W. DAB2IP plays important clinical significance and correlates with immune infiltration in renal cell carcinoma. Technol Cancer Res Treat. 2020;19:1533033820936682.
doi pubmed - De Florian Fania R, Bellazzo A, Collavin L. An update on the tumor-suppressive functions of the RasGAP protein DAB2IP with focus on therapeutic implications. Cell Death Differ. 2024;31(7):844-854.
doi pubmed - Kannan B, Pandi C, Pandi A, Jayaseelan VP, Arumugam P. Triggering receptor expressed in myeloid cells 1 (TREM1) as a potential prognostic biomarker and association with immune infiltration in oral squamous cell carcinoma. Arch Oral Biol. 2024;161:105926.
doi pubmed - Mo CK, Liu J, Chen S, Storrs E, Targino da Costa ALN, Houston A, Wendl MC, et al. Tumour evolution and microenvironment interactions in 2D and 3D space. Nature. 2024;634(8036):1178-1186.
doi pubmed - Filippou PS, Karagiannis GS, Constantinidou A. Midkine (MDK) growth factor: a key player in cancer progression and a promising therapeutic target. Oncogene. 2020;39(10):2040-2054.
doi pubmed - Zhang Y, Zuo C, Liu L, Hu Y, Yang B, Qiu S, Li Y, et al. Single-cell RNA-sequencing atlas reveals an MDK-dependent immunosuppressive environment in ErbB pathway-mutated gallbladder cancer. J Hepatol. 2021;75(5):1128-1141.
doi pubmed - Shi N, Chen S, Wang D, Wu T, Zhang N, Chen M, Ding X. MDK promotes M2 macrophage polarization to remodel the tumour microenvironment in clear cell renal cell carcinoma. Sci Rep. 2024;14(1):18254.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.