| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 3, June 2025, pages 311-316
Differentiating Malignant and Healthy Areas in Isolated Kidney Samples Through Infrared Visualization Techniques
Besarion Partsvaniaa, d, Tamaz Sulaberidzea, Alexandre Khuskivadzeb, Sophio Abazadzeb, Teimuraz Gogoladzea, Nutsa Khuskivadzec
aDepartment of Bio-Cybernetics, Institute of Cybernetics, Georgian Technical University, Tbilisi, Georgia
bDepartment of Urology, Georgia-Israel Joint Clinic “Gidmrdi”, Tbilisi, Georgia
cDepartment of Oncology, Tbilisi State Medical University, Tbilisi, Georgia
dCorresponding Author: Besarion Partsvania, Institute of Cybernetics, Georgian Technical University, Tbilisi 0186, Georgia
Manuscript submitted April 16, 2025, accepted May 30, 2025, published online June 14, 2025
Short title: Infrared Imaging to Distinguish Kidney Tumors
doi: https://doi.org/10.14740/wjon2593
| Abstract | ▴Top |
Background: Because partial nephrectomy (PN) may remove malignant tissue while maintaining kidney function, it is currently the gold standard for nephrectomy. However, the blood arteries that supply the kidney are clamped at the start of the procedure. The most common method for evaluating surgical margins during PN is intraoperative frozen section (FS) evaluation. Its long duration and high false-negative rate question its reliability and efficacy. This encouraged us to search for a much quicker and easier method.
Methods: The infrared (IR) imaging approach uses the differences in optical density between tumor and healthy tissue to create the sharp contrast in the IR images. The cancerous kidneys were examined after a radical nephrectomy. Following the removal of the cancerous tissue and some of the surrounding healthy tissue, the samples were examined using the IR method. For the IR analysis, we created specific software. Following that, tissue samples taken from both healthy and malignant areas were subjected to a histomorphological analysis.
Results: Experiments showed that malignant tissue appeared as areas of high blackness in the IR picture, while healthy tissue appeared as areas of high illumination. Our software highlighted the areas of the IR image that were associated with the healthy and malignant portions, computed their average brightness, and calculated the ratio of the average illumination (RAI) of the malignant area to that of the healthy area. RAI is an interval of numbers obtained as a result of dividing the average brightness of all dark areas in all examined samples by all light areas of all examined samples. The 95% probability interval for RAIs taking place, which ranged from 0.25 to 0.41, was calculated. The location of the malignancy was then identified by a histomorphological examination. The compliance between histomorphological results and the outcomes of IR examination was confirmed in all cases.
Conclusions: The IR imaging technique offers significant promise for improving the accuracy and efficiency of margin assessment during kidney cancer surgeries. The IR imaging technique can provide immediate feedback on the tumor boundaries, which could potentially reduce the duration of warm ischemia during surgery. Subsequent investigations should be focused on verifying the technology in further clinical trials and investigating its integration into the surgical process, which could result in its acceptance as a standard instrument for intraoperative decision-making in kidney cancer operations.
Keywords: Cancer; Kidney; Partial nephrectomy; Infrared imaging
| Introduction | ▴Top |
Globally, the incidence of kidney cancer has risen by 2% in the last 20 years. Surgery is often the primary treatment for kidney cancer, especially for localized tumors. The goal is to remove the tumor entirely, which can significantly enhance survival rates and reduce the risk of cancer recurrence. The two primary surgical techniques for kidney cancer are partial nephrectomy (PN), also referred to as nephron-sparing surgery, which removes only the tumor and a small margin of healthy tissue, and radical nephrectomy (RN), which removes the entire kidney along with surrounding tissues. One significant advantage of PN is the preservation of the kidney and its function.
After the cancerous portion along with some healthy portion is removed from the kidney during the operation, the material is sent for examination, where it is frozen for express histomorphological examination. This intraoperative diagnosis is known as the frozen section (FS) diagnosis [1, 2]. FS analysis is primarily utilized to evaluate the surgical margins during PN and to determine whether there remains any tumor in the nephrectomy site. FS aids surgeons in making real-time decisions during surgery, such as whether to proceed with further resection or adjust surgical strategy based on margin status [3].
Some surgeons express confidence in their ability to achieve complete resection without relying on FS, leading to varied practices regarding its use. This reflects a broader debate within the surgical community about when and how often to utilize intraoperative FS.
The kidney’s blood arteries are clamped at the start of the procedure. Research indicates that the duration of warm ischemia is crucial. Prolongation of duration of ischemia is associated with a higher likelihood of renal complications [4, 5]. Some complications can be postoperative renal failure and chronic kidney disease. Studies indicated that each additional minute of warm ischemia increases the likelihood of these complications, with a critical threshold identified at around 25 min, beyond which the risk escalates significantly [6, 7].
Concerning the other methods, fluorescence imaging offers high-resolution, molecularly targeted visualization useful for intraoperative guidance but requires contrast agents and has limited penetration depth. Raman spectroscopy provides detailed molecular information but is limited by weak signals and shallow tissue penetration. Optical coherence tomography (OCT) offers high-resolution structural imaging but limited functional data.
These circumstances motivated us to seek a novel method that is significantly faster than FS analysis and simplifies the distinction between the benign and malignant components of a resected kidney.
| Materials and Methods | ▴Top |
Kidney specimens taken following an open RN were used in 32 investigations. In our experiments, tumor characteristics are shown in Table 1.
 Click to view | Table 1. Tumor Characteristics |
After being informed, all patients whose kidney samples were used in these investigations provided written consent. The Independent Local Ethical Commission of the Georgia-Israel Joint Clinic “Gidmedi” approved the study protocol (ILEC ID No. 35).
After extraction, the entire kidney tumor was removed along with the part of the surrounding healthy tissue. Then the infrared (IR) technique was used to investigate this specimen.
A charge-coupled device (CCD) camera IR-1000 (DAGE-MTI, spectral response 400 - 1,100 nm, resolution 570 TVL 560 TVL, sensitivity 0.05 fc (0.5 lux) on sensing area, minimum illumination 0.00035 fc (0.0035 lux)), a holder for the specimen, and an IR irradiation source (LED 850, irradiating angle 130, radiant intensity 1 mW/sr at 20 mA nm invisible to humans) constituted the experimental setup (Fig. 1). IR radiation emitted by LEDs penetrates the investigated tissue. Following their passage through the tissue, IR rays carry information regarding the tumor-induced inhomogeneity of optical density within the kidney tissue. The rays fall on the IR-sensitive matrix of the CCD camera. The software, which was developed by us, converts the electrical signals from the CCD camera into visual 2D images or IR images after the output of the camera is connected to the computer. The custom software was previously utilized in our earlier study on prostate cancer visualization using IR imaging, where we evaluated its cancer detection performance by comparing IR images with pathology-confirmed ground truth [8, 9]. The software incorporates t-distribution statistics to calculate 95% confidence intervals automatically. In those studies, the software achieved an average sensitivity of 89% and a specificity of 91%. Software was validated by verifying the normality of the data distribution using Shapiro-Wilk tests. The software’s statistical computations were cross-checked against standard statistical packages (R), confirming accuracy.
 Click for large image | Figure 1. Setup of the experiment. Arrows are used to show the CCD camera and LEDs. Between them lies biological tissue. To prevent the influence of daylight, experiments were conducted in complete darkness. |
In the present work, we have not yet determined sensitivity and specificity, as this will be addressed in future investigations. However, since the same software is being used as in the prostate cancer imaging study, we consider its validation to be at least partially established for kidney tumor imaging.
We analyzed the IR images of the kidney specimens using the software that we created. The software determines the ratio of illumination intensities at areas on the IR image that correspond to malignant and healthy tissues using the following method. Depending on the illumination (brightness) of each dot in the IR image, the software assigns a number between 0 and 255. A dot that is maximally dark is given the number 0, and a point with the highest brightness is given the number 255. On the IR image, the areas with the highest brightness are outlined and the same code, for example, 0, is assigned to them. We assume these brighter areas correspond to non-cancerous tissue. Next, the darker areas are outlined, which are suspected to be malignant, and a different code, such as 1, is assigned to them. Our assumptions about whether an outlined area is malignant or healthy are later verified microscopically, and these are usually confirmed.
The software measures the light intensities at each point on the IR image, which represents both malignant and healthy tissues. After this, we give command to software to save this information in the memory. We repeat this process for every subsequent sample.
After collecting results from 32 different kidney samples (all stored in the software), we instructed the software to “analyze image.” As a result, the software then calculates the average brightness for all dark areas and all bright areas. After this step, the software computes the ratio of these numbers. This produces a range of values. This means that the software calculates the interval of values for above mentioned ratios with 95% of probability, called 95% confidence interval.
When we analyzed a new, unknown specimen using the same software procedures, the software gives a single number instead of an interval. If this obtained number falls within the established confidence interval, we can state with 95% probability that the specimen is cancerous.
Complete time of sample’s IR analyzed on out setup requires only 5 - 6 min, and thereby, in our opinion, this feature has an advantage over FS method, if it will be adopted as a routine method.
After IR investigation, the specimen was processed for histomorphological investigations with well-known procedures. All slides for microscopic investigations were reviewed independently by two board-certified pathologists, blinded to the imaging results.
All these procedures give the opportunity to know which part of the kidney belongs to the investigated microscope slice.
For histomorphological investigations, an Opto-Edu microscope (RoHS) with the microscope industrial digital camera E31SPM12000KPA was used.
| Results | ▴Top |
Experiments showed that healthy kidney tissue has a uniform optical density, meaning that its optical properties are consistent. The intensity of IR rays coming from this tissue is also steady. Consequently, the IR image of this area appears with even brightness across the entire area. This finding is depicted in Figure 2.
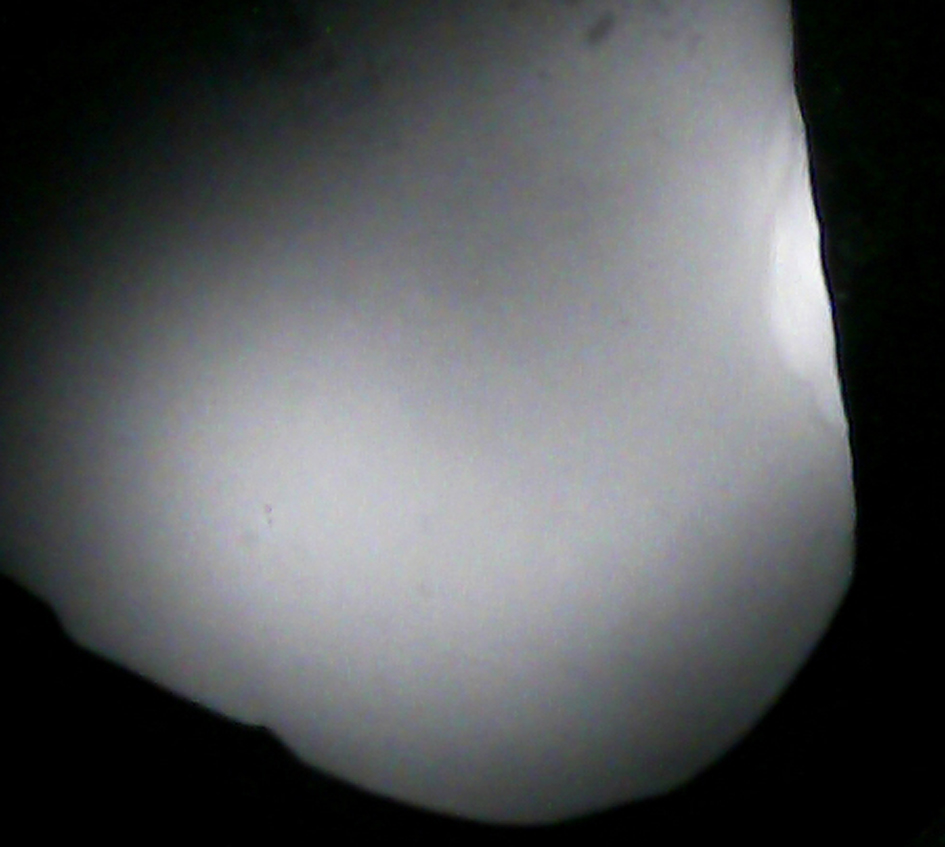 Click for large image | Figure 2. Infrared image of a small section of a non-cancerous kidney sample, showing uniform brightness throughout all areas. |
As our earlier studies showed, if the tissue is heterogeneous (containing both cancerous and healthy cells), the optical densities of these different tissues will vary, leading to differences in the intensity of the IR light that passes through them. Specifically, tumor tissue has a higher optical density than healthy tissue, resulting in a lower intensity of IR rays after they pass through the tumor compared to healthy tissue [9-11]. The same situation exists in the case of kidney tumors. Experiments revealed that the optical density of cancerous kidney tissue is much higher than that of healthy tissue. Therefore, the cancerous tissue is observed as areas of high darkness, while the healthy tissue is observed as areas with high illumination in the IR image, as illustrated in Figure 3. Here we see both the excised kidney tumor alongside nearby healthy tissue and the IR image of this specimen. In the IR image, the tumor corresponds to an area with low brightness, while the healthy tissue corresponds to an area with much higher brightness. The margins between cancerous and healthy tissues are also well-defined (Fig. 3).
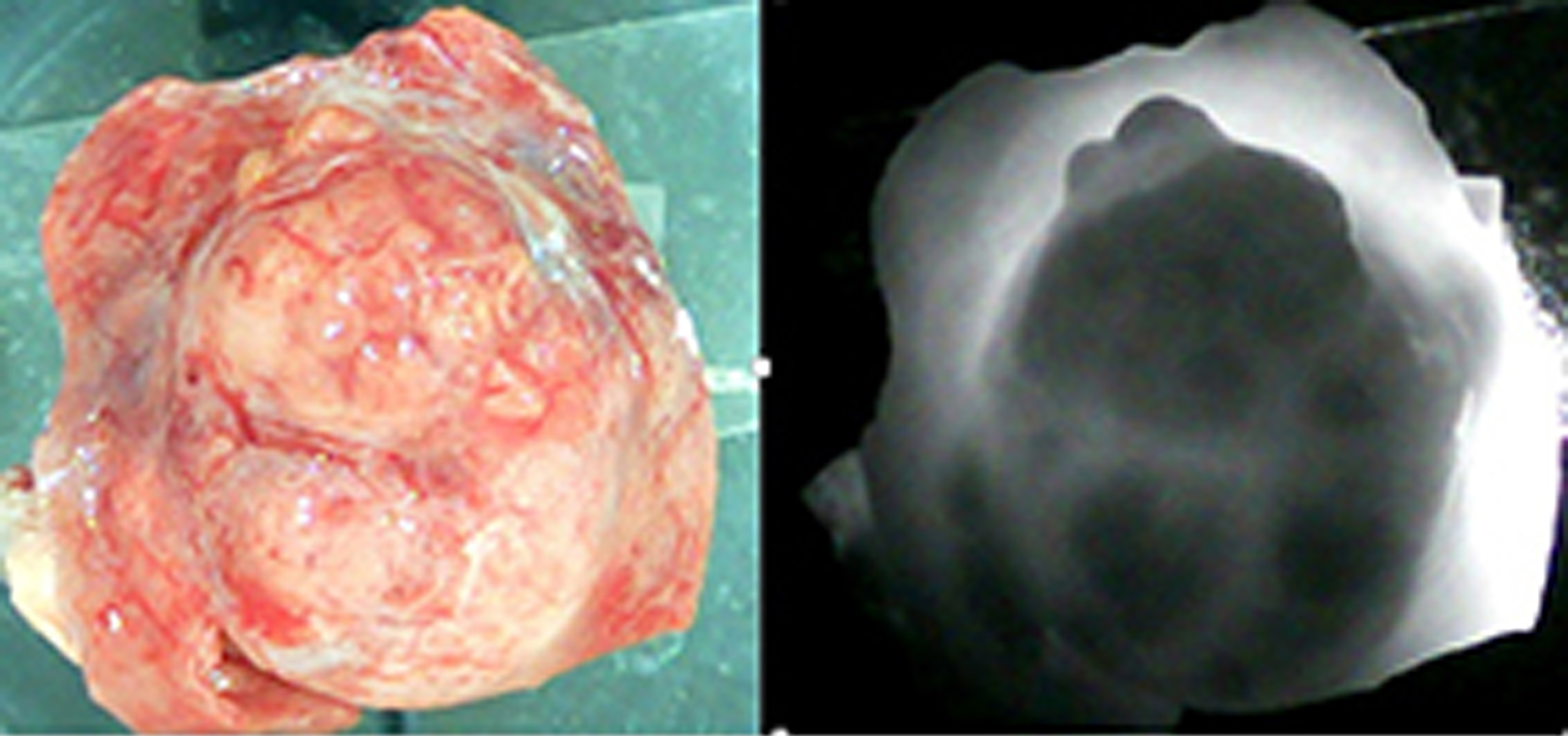 Click for large image | Figure 3. The left side shows a portion of cancerous tissue alongside nearby healthy tissues, extracted from the entire kidney after a radical nephrectomy. The right side shows the infrared image of this specimen. The dark areas correspond to cancerous tissue. The light areas correspond to the healthy portions of the tissue. The margins between the malignant and non-cancerous tissues are well-defined. |
The software calculated the interval for the ratio of the malignant area’s mean illumination intensity to the non-cancerous area’s mean illumination intensity for all specimens with a 95% probability, which ranges from 0.25 to 0.41. If we obtain a fresh, unidentified kidney sample with a tumor following these investigations, we will process it using our software. The average illumination ratio between the malignant and healthy areas will be computed. If the result falls between 0.25 and 0.41, we may say with 95% certainty that we can distinguish between tumor and healthy tissues.
For testing our findings, we referred to histomorphological analyses of tissues, for which these results were compared to the results of IR studies. Figure 4 shows the histomorphological images of specimens obtained from tumorous and healthy areas of the specimen shown in Figure 3.
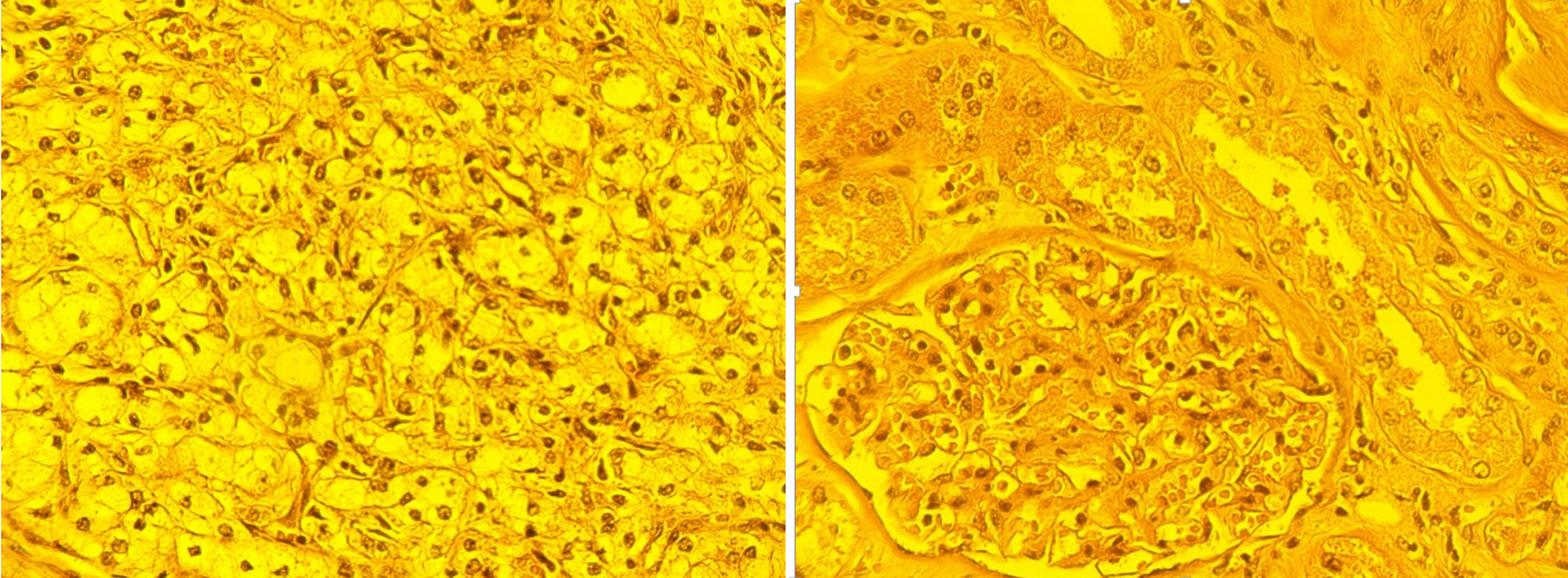 Click for large image | Figure 4. Histomorphological images. Left image corresponds to dark area in the Figure 3. The image shows large, polygonal cells with clear cytoplasm and distinct cell borders. The nuclei are centrally located and appear somewhat irregular. The normal kidney architecture is lost. There is no clear organization into tubules or glomeruli. The tissue is highly cellular, with cells appearing crowded and disorganized. There is minimal supporting connective tissue visible between the tumor cells. Right image corresponds to light area in Figure 3 (healthy kidney tissue). Key features: The round structure in the center is a glomerulus, a normal component of kidney tissue involved in blood filtration. Surrounding the glomerulus are well-organized tubules, lined by cuboidal epithelial cells. The cells are uniform, with regular nuclei and well-defined borders. There is a balanced amount of connective tissue providing structural support (× 400, caliber 100 µm). |
In Figure 4, areas determined as malignant and healthy by IR analysis are confirmed by histomorphological findings. Namely, in the left image, a histologic image of clear cell kidney cancer is shown, while in the right image, a histologic image of healthy tissue is shown. There are no tumor lesions. Thus, histomorphological analysis confirms that the areas identified by IR investigation as cancerous or healthy were indeed cancerous and healthy, respectively.
| Discussion | ▴Top |
The growing incidence of kidney cancer globally and the pivotal role of surgical interventions, particularly nephrectomy, in the treatment of this malignancy underscore the need for advancements in surgical techniques and diagnostic technologies. The current gold standard in nephrectomy is PN, due to its ability to preserve kidney function while removing cancerous tissue. However, this approach is challenged by the need for real-time confirmation of surgical margins to ensure complete tumor excision and minimize the risk of recurrence. Intraoperative FS examination is the prevailing technique for assessing surgical margins during PN. Yet its limitations, such as the high time duration, raise questions about its efficacy in terms of ischemia during surgery and ensuring optimal patient outcomes.
Our study aimed to explore a novel approach to the assessment of margins between tumor and healthy tissues using IR imaging.
The results of our study reveal significant potential in using IR imaging to differentiate between cancerous and non-cancerous kidney tissues with remarkable clarity. The IR imaging technique utilizes optical density differences between tumor and healthy tissues, which results in sharp contrast in the IR images. This contrast aligns with histological findings, where cells of a tumor tissue show an entirely different appearance in comparison with healthy cells. Results of IR investigations of kidney specimens are in agreement with previous studies [11, 12] that identified the optical density differences between cancerous and non-cancerous tissues.
The integration of IR imaging into the existing surgical workflow will not be a potential challenge because learning to interpret the IR images quickly and accurately during the operation is easy. Any participant in the operation can become familiar with the new technology. It may develop into an additional tool that improves the surgeon’s capacity for making judgments in real time while performing surgery. By providing real-time, accurate identification of tumor boundaries, it has the potential to reduce the incidence of positive surgical margins and improve long-term patient outcomes. Besides, the cost of the technology is very low.
The speed of this IR imaging method (5 - 6 min) is one of its greatest advantages. Unlike the time-consuming process of preparing and examining FSs, the IR imaging technique will be able to provide immediate feedback on tumor boundaries. This capability can potentially reduce the duration of warm ischemia during surgery, which is crucial for improving postoperative outcomes.
While the IR imaging method demonstrated promising results in differentiating between malignant and healthy tissue, several important considerations must be addressed before it can be potentially implemented. While our preliminary results are encouraging, the IR imaging technique is still in the early stages of research and development. Further validation in larger, prospective clinical studies is required before it can be considered for use in the operating room. The sensitivity/specificity of the IR technique needs to be further validated in larger studies to ensure its reliability and accuracy across various patient populations and tumor characteristics.
Although the IR imaging technique shows promise in differentiating malignant from healthy kidney tissues in excised specimens, its direct application in the intraoperative surgical setting is currently limited. The method has not yet been validated for real-time, in situ use during surgery, and further studies are required to assess its feasibility, reliability, and integration into the surgical workflow.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by Shota Rustaveli National Science Foundation of Georgia (SRNSFG), grant number No. FR-22-195. Project title: “Development of the new infrared imaging method for avoiding of cancer recurrence after radical prostatectomy and partial nephrectomy.”
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
After being informed, all patients whose kidney samples were used in these investigations provided written consent.
Author Contributions
Besarion Partsvania performed the infrared investigations. Tamaz Sulaberidze was responsible for software development and conducted the statistical calculations. Alexandre Khuskivadze and Sophio Abazadze performed the surgical operations. Teimuraz Gogoladze and Nutsa Khuskivadze carried out the histomorphological investigations. All authors contributed to the study design, data interpretation, manuscript preparation, and approved the final version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Breda A, Stepanian SV, Liao J, Lam JS, Guazzoni G, Stifelman M, Perry K, et al. Positive margins in laparoscopic partial nephrectomy in 855 cases: a multi-institutional survey from the United States and Europe. J Urol. 2007;178(1):47-50; discussion 50.
doi pubmed - Ceylan Y, Gunlusoy B. Surgeons’ preferences and practice patterns regarding intraoperative frozen section during partial nephrectomy. Urol Oncol. 2015;33(1):52.
doi pubmed - Carbonara U, Amparore D, Gentile C, Bertolo R, Erdem S, Ingels A, Marchioni M, et al. Current strategies to diagnose and manage positive surgical margins and local recurrence after partial nephrectomy. Asian J Urol. 2022;9(3):227-242.
doi pubmed pmc - Antonelli AD, Cindolo L, Sandri M, Veccia A, Annino F, Bertagna F, Di Maida F, et al. The role of warm ischemia time on functional outcomes after robotic partial nephrectomy: a radionuclide renal scan study from the clock randomized trial. World J Urol. 2023;41(5):1337-1344.
doi pubmed pmc - de la Parra I, Gomez Rivas J, Serrano A, Vives R, Gutierrez Hidalgo B, Hermida JF, Ibanez L, et al. Ischemia time in partial nephrectomy: to rush really matters? Mini-invasive Surg. 2024;8:16-29.
- Drake R. Limit warm ischemia to 25 min during partial nephrectomy. Nat Rev Urol. 2010;7(8):422-430.
- Lane BR, Gill IS, Fergany AF, Larson BT, Campbell SC. Limited warm ischemia during elective partial nephrectomy has only a marginal impact on renal functional outcomes. J Urol. 2011;185(5):1598-1603.
doi pubmed - Abazadze S, Partsvania B, Khuskivadze A. Infrared visualization of the prostate cancer. G Biomed N. 2023;1(1):26-28.
- Partsvania B, Petriashvili G, Fonjavidze N. Possibility of using near infrared irradiation for early cancer diagnosis. Electromagn Biol Med. 2014;33(1):18-20.
doi pubmed - Partsvania B, Sulaberidze T, Khuskivadze A, Abazadze S. Prostate cancer diagnostics modeling using the infrared imaging method. Exp Oncol. 2024;46(3):268-272.
doi pubmed - Khuskivadze A, Partsvania B, Kochiashvili D. Visualization of human prostate cancer using infrared radiation. Urology. 2014;84:S304.
- Abazadze S, Khuskivadze A, Kochiashvili D, Partsvania B. Dependence of prostate tissue permeability on the wavelength of radiation in the infrared range of the spectrum. Georgian Med News. 2021;321:111-115.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.