| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 000, Number 000, July 2025, pages 000-000
Clinicopathological Features of HER2 Expressing Lobular Carcinoma of Breast
Majd Khadera, Bayan Maraqaa, Hikmat Abdel-Razeqb, Khaled Halahlehb, Maher Sughayera, c
aDepartment of Pathology and Laboratory Medicine, King Hussein Cancer Center, Amman, Jordan
bDepartment of Medical Oncology, King Hussein Cancer Center, Amman, Jordan
cCorresponding Author: Maher Sughayer, Department of Pathology and Laboratory Medicine, King Hussein Cancer Center, Amman 11941, Jordan
Manuscript submitted April 8, 2025, accepted June 16, 2025, published online July 8, 2025
Short title: HER2-Expressing Lobular Carcinoma
doi: https://doi.org/10.14740/wjon2588
| Abstract | ▴Top |
Background: Invasive lobular carcinoma (ILC) accounts for approximately 10% of invasive breast carcinomas and is the most common special subtype. Most ILCs express estrogen receptors (ERs) and progesterone receptors (PRs) but typically lack ERBB2 (human epidermal growth factor receptor 2 (HER2)) overexpression. HER2-positive ILC is rare, understudied, and often linked to aggressive clinical and histopathologic features. This study aimed to examine the clinicopathologic characteristics of HER2-positive ILC to ensure proper classification and management.
Methods: A retrospective review was conducted on 48 cases, including 28 HER2-positive ILC and 20 pleomorphic invasive lobular carcinoma (p-ILC) cases without HER2 overexpression. Histological features assessed included nuclear pleomorphism, signet ring cell morphology, and apocrine features. Hormone receptor status and clinical outcomes were also analyzed.
Results: All HER2-positive ILC cases exhibited at least one pleomorphic histological feature. Hormone receptor positivity was lower in HER2-positive ILC compared to p-ILC without HER2 overexpression. However, overall survival did not significantly differ between the two groups.
Conclusion: HER2 overexpression in ILC is frequently associated with pleomorphic features. p-ILC, regardless of HER2 status, portends a worse prognosis. Identifying these features in HER2-positive ILC and classifying them as pleomorphic lobular carcinoma, a more aggressive ILC variant, is crucial for closer patient follow-up.
Keywords: Invasive lobular carcinoma; Breast cancer; HER2 overexpression; Pleomorphic features; Hormone receptors; Prognosis
| Introduction | ▴Top |
Breast cancer is the most common malignant tumor in women worldwide and it is the leading cause of death from cancer in women as well according to the 2022 updated estimates from the International Agency for Research on Cancer (IARC) [1]. Breast cancer represents a heterogeneous group of diseases with distinct histological subtypes, each carrying unique clinical and molecular features [2]. Molecular profiling has shown the range of heterogeneity and classified it into four main subtypes: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-enriched, and basal subtypes. In recent years, considerable attention has been directed towards the characterization and understanding of invasive lobular carcinoma (ILC), which is considered the most common special subtype of invasive breast carcinoma [3]. Five to fifteen percent of all invasive breast cancers are ILCs, and incidence rates of ILC have significantly increased during the past 20 years [4].
The most characteristic feature of ILC is the loss of the cell adhesion protein E-cadherin, leading to the typical discohesive cellular morphology recognizable by the predominance of single cells. This breast cancer has two main histological variants that are well-known and apparently have different biological behaviors: the classical and pleomorphic variants [4]. While the majority of ILC cases express estrogen receptors (ERs) and progesterone receptors (PRs), they typically lack ERBB2 (HER2) overexpression, with only a small subset exhibiting HER2 positivity ranging from 1% to 11% [5]. A previous study by the authors revealed that ILC with HER2 overexpression constituted 3.6% of all ILC cases, emphasizing the clinical significance of HER2 positivity in this subtype [6]. In addition to its clinical implications, the characterization of HER2-positive ILC holds significant prognostic and therapeutic relevance. Given the association between HER2 overexpression and aggressive tumor behavior in other breast cancer subtypes, it is imperative to ascertain whether HER2 positivity confers a similar adverse prognostic impact in the context of ILC.
Notably, ILCs with HER2 overexpression are infrequent and are often associated with non-standard histological variants, including the more aggressive pleomorphic invasive lobular carcinoma (p-ILC) [7].
According to the WHO Classification of Breast Tumors, p-ILC is defined as a variant of ILC characterized by markedly pleomorphic nuclei (> 4 times the size of lymphocytes/equivalent to that of high-grade ductal carcinoma in situ, with or without apocrine features) and increased mitotic activity, typically while retaining the discohesive growth pattern and loss of E-cadherin typical of ILC. While features such as apocrine differentiation, signet ring morphology, and histiocytoid changes may coexist with pleomorphic morphology, they are not, in themselves, diagnostic of the pleomorphic variant.
In contrast to classic invasive lobular carcinoma (c-ILC), p-ILC has increased cellular atypia and significant clinical implications due to its aggressive presentation, which is often characterized by larger tumor size, higher histological grade, and advanced stage at diagnosis [8, 9]. Furthermore, because pleomorphic lobular carcinoma (PLC) is a rare form of breast cancer, there is currently insufficient evidence to develop guidelines for its specific management. p-ILC is associated with a poorer prognosis when compared to other ILC variants. It has been reported that patients with p-ILC were four times more likely to experience recurrence than patients affected by classic variant. In addition, they tend to have lower rates of ER positivity and higher rates of HER2 expression [4].
In this research article, we aimed to investigate certain histopathologic features of HER2-positive ILC to ascertain whether they fulfill the criteria of p-ILC, a more aggressive variant of ILC and to compare the clinicopathologic characteristics of HER2-positive ILC with both c-ILC and p-ILC without HER2 overexpression. Through a multidisciplinary approach encompassing histopathological analysis, immunohistochemical (IHC), and clinical correlation, we aimed to unravel the intricate complexities of HER2-positive ILC and pave the way for more effective therapeutic interventions and improved patient outcomes.
| Materials and Methods | ▴Top |
This study was approved by the Institutional Review Board (IRB) of King Hussein Cancer Center, and all research was conducted in accordance with relevant guidelines and regulations. The reporting of this study conforms to STROBE guidelines [10]. The study was conducted within the Department of Pathology at the same institution. All procedures complied with ethical standards, and patient confidentiality was strictly maintained throughout the study. Utilizing our surgical pathology archival system, we conducted a retrospective review of all cases (a total of 28 cases) diagnosed as c-ILC with HER2 overexpression. The medical records, the original hematoxylin and eosin (H&E)-stained slides, and the HER2-immuostained slides were retrieved and reviewed. HER2 IHC staining was originally done using the rabbit monoclonal anti-HER2 antibody clone 4B5 on the Ventana Benchmark/Ultra platform (Roche/Ventana Biosystems, Tucson, AZ). The HER2 scoring was based on the 2013 College of American Pathologists (CAP)/American Society of Clinical Oncology (ASCO) guidelines, where positive expression is defined by either IHC 3+ score in which tumor cells display complete, intense membranous staining in > 10% of tumor cells or 2+ score and fluorescence in situ hybridization (FISH) amplified [11].
In addition, we also retrieved cases diagnosed as PLC without HER2 overexpression. All cases were originally diagnosed between 2008 and 2022.
The cohort was selected to focus on two specific groups: 1) cases initially diagnosed as c-ILC with confirmed HER2 overexpression; and 2) cases diagnosed as p-ILC without HER2 overexpression. This selection was based on availability within our institutional database and the intent to explore the relationship between HER2 status and pleomorphic morphology. No additional cases of HER2-negative c-ILC or HER2-positive p-ILC were identified beyond those already reclassified during histological review.
Histopathological analysis involved the retrieval of H&E slides, with a focus on identifying any morphological deviations from the classical features of lobular carcinomas in the invasive lesions. Specifically, we searched nuclear pleomorphism, signet ring cell morphology, and apocrine features. This detailed examination aimed to reassess cases previously diagnosed as c-ILC, considering reclassification as PLC. Concurrently, we sought to compare the histopathologic features of these cases with an established diagnosis of PLC that did not exhibit HER2 overexpression against those that did. Furthermore, the study included a follow-up component, where clinical outcomes such as death from the disease and distant metastasis were assessed. Information on hormonal profile status was extracted from patients’ files, providing a comprehensive view of the clinical course of these cases.
Descriptive statistics and Chi-square calculations were used in the study to analyze the findings. A P value of < 0.05 was used as a significance threshold.
| Results | ▴Top |
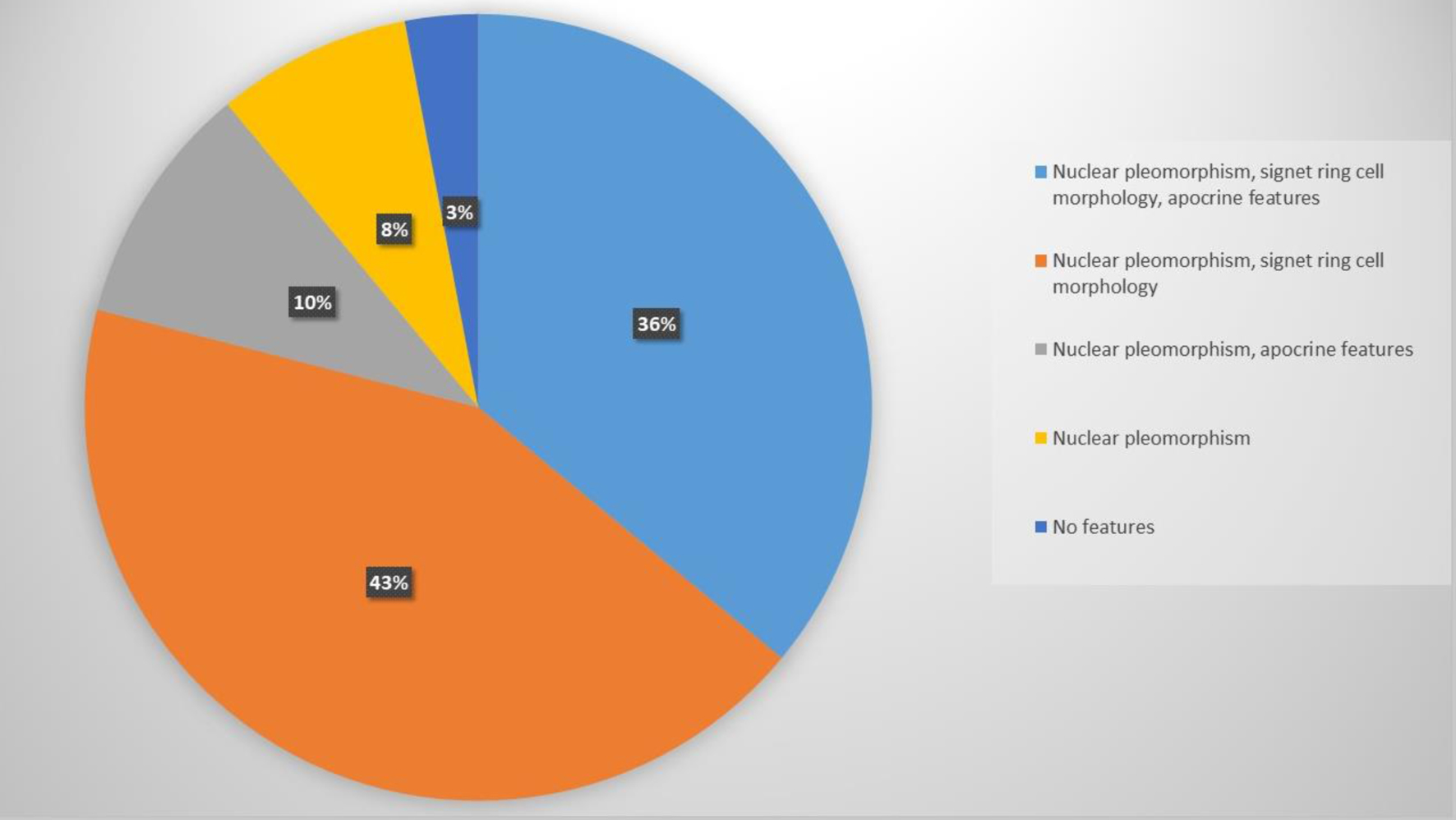
A total of 28 cases previously diagnosed as c-ILC overexpressing HER2 underwent a detailed histopathologic review to ascertain the presence of specific criteria. Table 1 summarizes their demographics. Twenty-five cases were HER2-positive by IHC and three cases were HER2-positive by FISH. Among these cases, 10 exhibited all three histopathologic features studied (nuclear pleomorphism, signet ring cell morphology, and apocrine features), 12 displayed nuclear pleomorphism and signet ring cell morphology, three demonstrated nuclear pleomorphism and apocrine features, two showed pleomorphism only, and one did not exhibit any of the studied features (Figs. 1 and 2).
 Click to view | Table 1. Demographics of HER2+ ILC |
 Click for large image | Figure 1. Histological features of HER2-positive invasive lobular carcinoma. HER2: human epidermal growth factor receptor 2. |
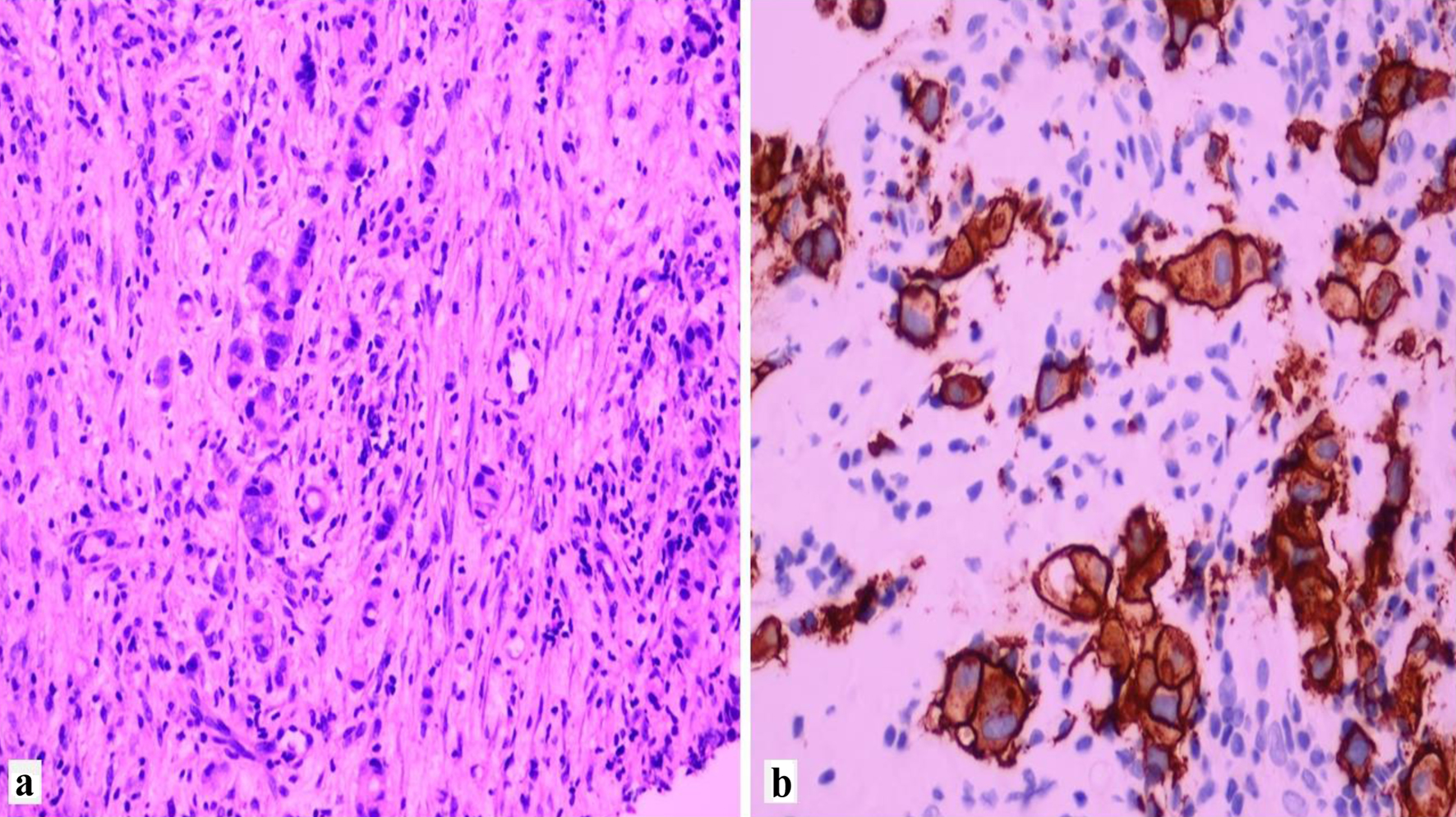 Click for large image | Figure 2. Example of invasive lobular carcinoma overexpressing HER2. (a) H&E × 40. (b) HER2 immunostain showing complete membranous staining, score +3. HER2: human epidermal growth factor receptor 2; H&E: hematoxylin and eosin. |
All 28 patients had available ER and PR results. Twenty-two cases were positive for ERs (78.6%), while 16 cases (57.1%) were positive for PRs.
In parallel, 20 cases previously diagnosed as PLC without HER2 overexpression were reviewed for shared histopathologic features. The results demonstrated comparability, with 16 cases exhibiting all three features in varying proportions. Interestingly one of the cases demonstrated focal ductal morphology. Regarding hormone receptor status, 17 cases (85%) were positive for ERs, and 14 cases (70%) were positive for PRs. Figure 3 displays the comparison of ER and PR status between the two groups showing the HER2 expressing group to be less likely positive for ER and PR.
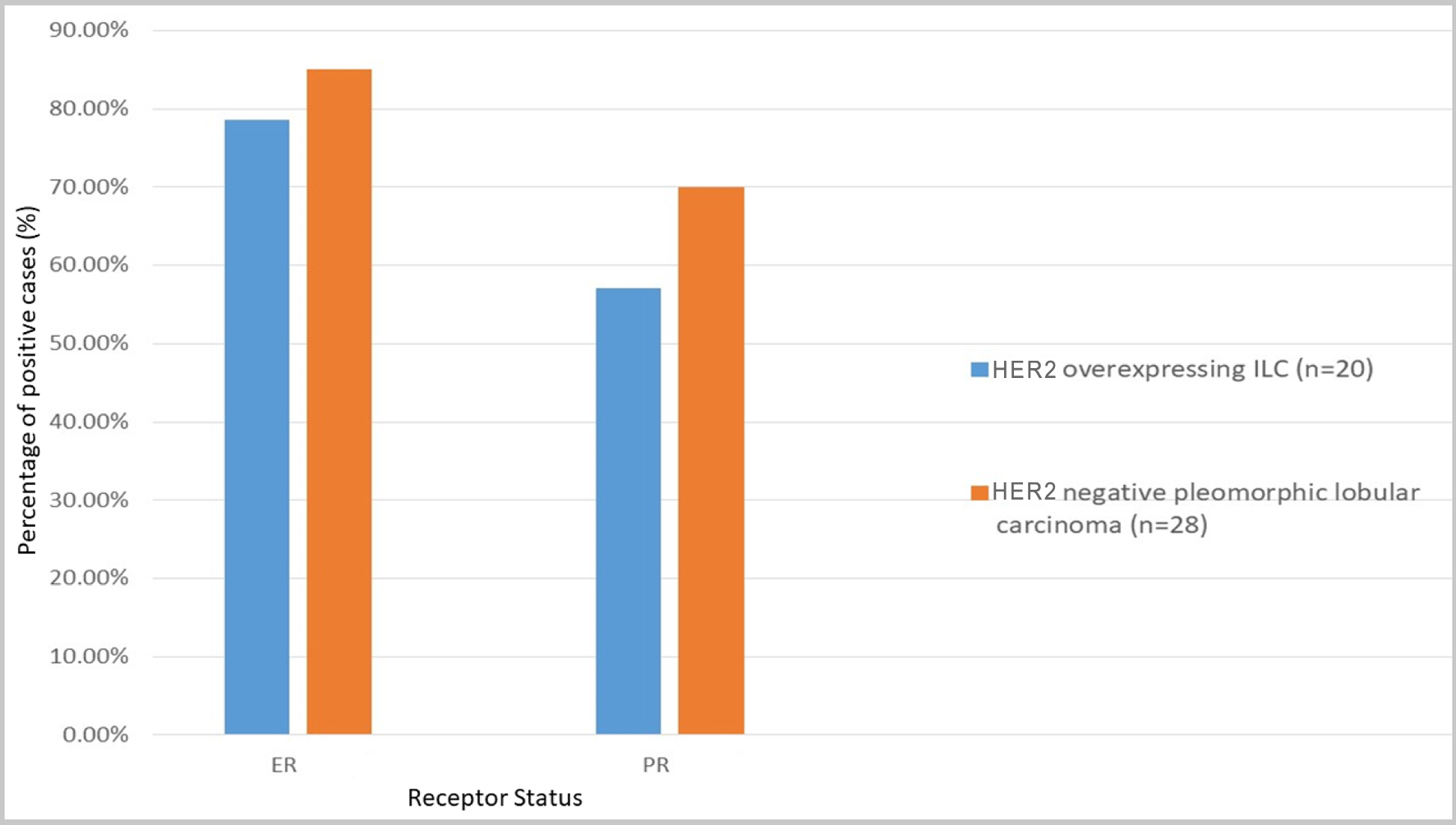 Click for large image | Figure 3. ER and PRs positive rate (%) comparison between HER2-negative and HER2-overexpressing ILC. ER: estrogen receptor; HER2: human epidermal growth factor receptor 2; ILC: invasive lobular carcinoma; PR: progesterone receptor. |
In the first group (ILC overexpressing HER2), follow-up data were available for an average duration of 51 months (ranging from 2 to 114 months). At 60 months, the overall survival (OS) rate was 40.7%. At time of last follow-up, among the 28 patients, seven were alive, four were alive with advanced disease (metastasis), and nine were deceased. Eight patients were lost to follow-up, with no current outcome data available (Table 2). Out of the seven patients who remained alive, three were assigned a pathologic stage of ypT0N0 (43%) at the time of resection.
 Click to view | Table 2. Comparison in Survival Status Between HER2-Negative p-ILC and HER2-Overexpressing ILC |
In the second group (PLC without HER2 overexpression), patients were followed up over an average duration of 21 months (ranging from 2 to 102 months). At the last follow-up, seven patients were deceased, two were alive with advanced disease, eight were alive with no recurrence or metastasis, and three were lost to follow-up. There was no significant difference in the survival between the two groups of patients as seen in Table 2 using the Chi-square test where the raw Chi-square values and the P value which were more than 0.05.
| Discussion | ▴Top |
HER2 gene amplification is seen in 20% to 30% of all breast cancers [12]. ILC that overexpresses HER2 is uncommon [6]. In some published studies, the prevalence of HER2 overexpression was somewhere between 0% and 10% [13, 14]. There is significant variability in HER2 status in PLC ranging from 15% to 30% in the literature, but most studies have shown that p-ILC rather than c-ILC is more likely to be associated with HER2 positivity [8, 15-17]. In one study carried out in Jordan to detect prevalence rates of ER, PR, and HER2 overexpression among Jordanian patients with breast cancer, it was found that out of a total of 1,185 patients, 84 patients were diagnosed with ILC, and out of these 84 patients, only three patients (3.6%) showed HER2 overexpression [6]. These findings collectively underscore the infrequency of HER2 overexpression in ILC, indicating that its occurrence may be linked to specific deviations from the classic histopathologic features characteristic of c-ILC.
In this study, we examined 48 cases, comprising 28 cases previously diagnosed as c-ILC with HER2 overexpression and 20 cases initially diagnosed as PLC without HER2 overexpression. The results showed that the histopathologic features we investigated were common in both groups, with comparable expression. Notably, all cases previously diagnosed as PLC exhibited the identified histopathologic features, and nearly all cases of HER2-positive ILC also displayed these characteristics (Fig. 1).
Nuclear pleomorphism, signet ring cell morphology, and apocrine features were studied. High nuclear grade, as reflected in nuclear pleomorphism, has been linked to HER2 overexpression in some studies [18, 19]. In line with those reports, this feature was present in all but one of the HER2-positive cases in our study.
The finding that most HER2-positive ILC cases originally classified as c-ILC exhibited pleomorphic features underscores a potential under-recognition of pleomorphism in this subtype. This reclassification was part of the study design and was based on standardized histopathologic criteria (including nuclear pleomorphism, apocrine differentiation, and signet ring morphology), reviewed by experienced breast pathologists. Rather than reflecting diagnostic error, this likely indicates morphologic overlap that challenges rigid classification. These findings highlight the importance of thorough histological reassessment, particularly when HER2 positivity is identified in ILC.
Regarding signet ring cell morphology and apocrine features, they were variably noted in the two groups with some cases showing only focal expression of the latter features. What can be concluded from this is that ILC that overexpresses HER2 almost always exhibits histological features that deviate from the classic morphology of c-ILC in which the cells are typically small and monomorphic and show minimum amount of cytoplasm [20].
The vast majority (80-95%) of ILCs exhibit ER positivity, classifying them as the luminal A subtype. This subtype is further characterized by PR positivity, HER2 negativity, and a low Ki67 proliferative index, indicating favorable prognosis [21].
Hormone receptor status in p-ILC exhibits noteworthy variability across published case series. While earlier studies reported a significantly lower ER/PR positivity rate (10-20%) compared to c-ILC, more recent research reveals hormone receptor profiles closer to those of c-ILC, ranging from 57% to 96% [15, 17, 22, 23]. This discrepancy likely reflects a combination of evolving diagnostic criteria, improved testing methods, and increased recognition of pleomorphic features. In this study, we concur with recent findings, with ER positivity at 78.6% and PR positivity at 57% in p-ILC cases.
Yu et al [18] studied ER and PR expression and their results showed that there was no difference in ER positivity between the HER2-positive and HER2-negative groups of ILC patients. However, inability to express PR was substantially correlated with HER2 overexpression, an observation that has been recognized in other studies as well [24]. In our study, among the 28 cases of HER2-overexpressing ILC, 78.6% expressed ER, while only 57.1% expressed PR. When comparing these percentages to those of p-ILC cases that did not overexpress HER2 (ER positivity: 85%, PR positivity: 70%), an association emerges, suggesting that HER2 overexpression in ILC is linked to decreased expression of hormone receptors, akin to findings in HER2-overexpressing invasive ductal carcinoma (IDC) [25, 26].
Another feature evaluated by Yu et al [18] that was associated with HER2 overexpression was histiocytoid morphology, which was strongly associated with HER2 overexpression, a finding that was not observed in our study but could be explained by the relatively small number of cases included. Nonetheless, the same study indicated that there is a significant overlap in apocrine features (observed in our study) and histiocytoid morphology and that the terms are used interchangeably in the literature.
The infrequent occurrence of HER2 overexpression in ILC raises the crucial question of whether the initial diagnosis of the lobular phenotype was accurate in all cases. Previous research has demonstrated that reassessing ILC cases with HER2 overexpression can reveal the presence of ductal structures, prompting reclassification as IDC [16]. In our study, however, all 48 cases diagnosed as ILC were classified as such based on the absence of E-cadherin membranous staining. This approach is supported by the high sensitivity and specificity of negative E-cadherin staining for diagnosing ILC, although one case showed focal ductal morphology but yet failed to show membranous E-cadherin staining [27]. While poorly differentiated IDC may exhibit decreased E-cadherin expression, complete loss of membranous staining, as observed in all our ILC cases, is uncommon in IDC according to published evidence [28].
It is important to note a key limitation of the study design: the two comparison groups differ in both HER2 expression status and histological subtype (classic vs. pleomorphic). This dual variation makes it challenging to isolate the independent effects of either variable. A more robust comparison would involve assessing HER2-positive versus HER2-negative tumors within a consistent histological background, or comparing pleomorphic and classic morphologies within the same HER2 status group. However, given the rarity of HER2-positive ILC and pleomorphic variants, especially in combination, such groupings were not feasible with our current sample size. Despite this limitation, the findings raise important hypotheses regarding the interplay between HER2 expression and pleomorphic histology, which warrant further investigation in larger cohorts.
When comparing the outcomes of the two groups, i.e., HER2-positive and HER2-negative ILC, we can conclude that there is no significant difference in the death rate (35% of HER2-negative ILC compared to 32% of HER2-positive ILC), and there is no statistically significant survival difference between the two groups as shown in Table 2. This finding indicates that pleomorphic morphology by itself without HER2 overexpression was linked to worse prognosis, a finding that is documented in literature [4, 29, 30].
It is important to note that survival analysis was conducted using a Chi-square test to compare categorical survival outcomes, which does not account for time-to-event variables such as follow-up duration. Ideally, a Kaplan-Meier analysis with log-rank testing would offer a more robust method for comparing OS. However, due to the small sample size and variable follow-up duration, this was not feasible. This represents a methodological limitation and should be addressed in future studies with larger, prospectively followed cohorts.
The 5-year OS rate in this cohort of patients is low at 32-35%. The rather poor outcome in our cohorts is most likely related to the fact that two-thirds of the patients were either metastatic or locally advanced at presentation. In addition, this unique cohort of HER2-positive ILC and p-ILC are known to be associated with poor outcome as has been previously shown by Iorfida et al [29]. In their study, they showed that non-classical ILCs have at least twice the risk of mortality as compared with c-ILCs and that HER2-positive ILCs were eight times more likely to develop distant metastasis. Added to this is the fact that only seven patients in our cohort received neoadjuvant combined anti-HER2/chemotherapy.
However, conclusions regarding prognosis and therapeutic response should be interpreted with caution due to the relatively small number of cases and the limited follow-up duration in this retrospective study. Larger, prospective studies with longer follow-up are necessary to validate these findings.
The data regarding outcome and management of HER2-positive ILC are controversial as in a recently published study we have found that patients with HER2-positive IDC experienced a survival advantage when receiving anti-HER2 therapy. However, in the case of HER2-positive ILC patients, the survival outcomes did not show improvement with anti-HER2 treatment, with their survival rates aligning closely with those of HER2-negative ILC patients [31]. Perhaps an alternative explanation for the findings in our study, indicating the lack of significant difference in survival rates, could be that patients with HER2-positive ILC might not derive the same benefits from anti-HER2 therapy as patients with HER2-positive IDC do. However, the advanced stage disease at presentation and the small sample size of our study warrant cautious interpretation of these findings and highlight the need for further research with larger cohorts to validate these observations.
The primary strength of this study lies in its focused histopathological reassessment of HER2-positive ILC cases, which revealed that nearly all such tumors previously diagnosed as c-ILC actually demonstrated pleomorphic features. This highlights a potential under-recognition of pleomorphic morphology in HER2-positive ILC and suggests that HER2 overexpression in ILC should prompt careful morphologic re-evaluation. The study contributes to the existing literature by underscoring the histological overlap and diagnostic challenges in this rare subgroup, with important implications for classification and clinical management. It also highlights that outcome of HER2+ ILC regardless of the histology features is the same.
This study focused primarily on histological morphology, hormone receptor status, and HER2 expression. Other potentially important clinicopathologic variables, such as Ki67 proliferation index, lymphovascular invasion (LVI), tumor multifocality, and use of endocrine or anti-HER2 therapy, were not consistently available across the included cases and therefore could not be comprehensively analyzed. These parameters are known to influence prognosis and treatment decisions and would enrich future analyses. We recommend that larger, prospective studies integrate these factors for a more holistic understanding of the behavior of ILC subtypes.
Conclusion
In conclusion, our study sheds light on the association between HER2 overexpression and pleomorphic features in ILC. HER2 positivity seems to influence hormone receptor expression but may not independently impact survival compared to the inherent aggressiveness of p-ILC. Despite the insights gained from this study, it is important to acknowledge the limitation posed by the small sample size, which may restrict the generalizability of the findings. Additionally, our study was conducted within a specific demographic or geographic region, which could limit the applicability of our results to broader populations. Furthermore, the retrospective nature of the study introduces potential biases and limitations inherent to retrospective data analysis, such as incomplete data or selection bias. Further research is warranted to refine diagnostic criteria and treatment strategies for this challenging breast cancer subtype.
Acknowledgments
This study was presented in abstract form at the United States and Canadian Academy of Pathology (USCAP) 2023 Annual Meeting, New Orleans, LA.
Financial Disclosure
This research received no specific grant from any commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that there is no conflict of interest.
Informed Consent
Informed consent was waived by the IRB due to the retrospective nature of the study.
Author Contributions
MK was responsible for acquisition of data and drafting of the manuscript. BM, KH, HA, and MS were responsible for critical revision of the manuscript. All authors reviewed the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263.
doi pubmed - Testa U, Castelli G, Pelosi E. Breast cancer: a molecularly heterogenous disease needing subtype-specific treatments. Med Sci (Basel). 2020;8(1):18.
doi pubmed - Rakha EA, Ellis IO. Lobular breast carcinoma and its variants. Semin Diagn Pathol. 2010;27(1):49-61.
doi pubmed - Wilson N, Ironside A, Diana A, Oikonomidou O. Lobular breast cancer: a review. Front Oncol. 2020;10:591399.
doi pubmed - Kee G, Tan R, Sultana R, Zaw M, Lian W, Tan K, Dent R, et al. HER2 positive rates in invasive lobular breast carcinoma: a study amongst 1,095 consecutive Asian patients. Annals of Oncology. 2018;29:ix1.
- Alsughayer AM, Dabbagh TZ, Abdel-Razeq RH, Al-Jussani GN, Alhassoon S, Sughayer MA. Changing trends in estrogen receptors/progesterone receptors/human epidermal growth factor receptor 2 prevalence rates among jordanian patients with breast cancer over the years. JCO Glob Oncol. 2022;8:e2100359.
doi pubmed - Huang X, Chen H, Ding Q, Robinson MK, Moseley TW, Bassett RL, Tang G, et al. Clinicopathological features of and neoadjuvant therapy for human epidermal growth factor receptor 2-positive classic invasive lobular carcinoma. Hum Pathol. 2021;117:51-59.
doi pubmed - Jung SP, Lee SK, Kim S, Choi MY, Bae SY, Kim J, Kim M, et al. Invasive pleomorphic lobular carcinoma of the breast: clinicopathologic characteristics and prognosis compared with invasive ductal carcinoma. J Breast Cancer. 2012;15(3):313-319.
doi pubmed - Al-Baimani K, Bazzarelli A, Clemons M, Robertson SJ, Addison C, Arnaout A. Invasive pleomorphic lobular carcinoma of the breast: pathologic, clinical, and therapeutic considerations. Clin Breast Cancer. 2015;15(6):421-425.
doi pubmed - von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349.
doi pubmed - Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997-4013.
doi pubmed - Iqbal N, Iqbal N. Human Epidermal Growth Factor Receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748.
doi pubmed - Bane AL, Tjan S, Parkes RK, Andrulis I, O'Malley FP. Invasive lobular carcinoma: to grade or not to grade. Mod Pathol. 2005;18(5):621-628.
doi pubmed - Vergine M, Brunelli M, Martignoni G, Brunello E, Miller K, Pecori S, Bersani S, et al. Suitability of infiltrative lobular breast carcinoma for anti-human epidermal growth factor receptor 2 treatment after the ASCO/CAP and 2009 St Gallen International Expert Consensus meeting. Histopathology. 2010;57(6):935-940.
doi pubmed - Varga Z, Zhao J, Ohlschlegel C, Odermatt B, Heitz PU. Preferential HER-2/neu overexpression and/or amplification in aggressive histological subtypes of invasive breast cancer. Histopathology. 2004;44(4):332-338.
doi pubmed - Hoff ER, Tubbs RR, Myles JL, Procop GW. HER2/neu amplification in breast cancer: stratification by tumor type and grade. Am J Clin Pathol. 2002;117(6):916-921.
doi pubmed - Monhollen L, Morrison C, Ademuyiwa FO, Chandrasekhar R, Khoury T. Pleomorphic lobular carcinoma: a distinctive clinical and molecular breast cancer type. Histopathology. 2012;61(3):365-377.
doi pubmed - Yu J, Dabbs DJ, Shuai Y, Niemeier LA, Bhargava R. Classical-type invasive lobular carcinoma with HER2 overexpression: clinical, histologic, and hormone receptor characteristics. Am J Clin Pathol. 2011;136(1):88-97.
doi pubmed - Zeillinger R, Kury F, Czerwenka K, Kubista E, Sliutz G, Knogler W, Huber J, et al. HER-2 amplification, steroid receptors and epidermal growth factor receptor in primary breast cancer. Oncogene. 1989;4(1):109-114.
pubmed - McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and 'omics. Breast Cancer Res. 2015;17(1):12.
doi pubmed - McCart Reed AE, Kalinowski L, Simpson PT, Lakhani SR. Invasive lobular carcinoma of the breast: the increasing importance of this special subtype. Breast Cancer Res. 2021;23(1):6.
doi pubmed - Jung HN, Shin JH, Han BK, Ko EY, Cho EY. Are the imaging features of the pleomorphic variant of invasive lobular carcinoma different from classic ILC of the breast? Breast. 2013;22(3):324-329.
doi pubmed - Rakha EA, van Deurzen CH, Paish EC, Macmillan RD, Ellis IO, Lee AH. Pleomorphic lobular carcinoma of the breast: is it a prognostically significant pathological subtype independent of histological grade? Mod Pathol. 2013;26(4):496-501.
doi pubmed - Kaptain S, Tan LK, Chen B. Her-2/neu and breast cancer. Diagn Mol Pathol. 2001;10(3):139-152.
doi pubmed - Xin LJJ, Eng LG. A review of invasive lobular carcinoma of the breast: Should it be treated like invasive ductal carcinoma? Integr Cancer Sci Therap. 2016;3(5).
- Fan Y, Wang Y, He L, Imani S, Wen Q. Clinical features of patients with HER2-positive breast cancer and development of a nomogram for predicting survival. ESMO Open. 2021;6(4):100232.
doi pubmed - Singhai R, Patil VW, Jaiswal SR, Patil SD, Tayade MB, Patil AV. E-Cadherin as a diagnostic biomarker in breast cancer. N Am J Med Sci. 2011;3(5):227-233.
doi pubmed - Moll R, Mitze M, Frixen UH, Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol. 1993;143(6):1731-1742.
pubmed - Iorfida M, Maiorano E, Orvieto E, Maisonneuve P, Bottiglieri L, Rotmensz N, Montagna E, et al. Invasive lobular breast cancer: subtypes and outcome. Breast Cancer Res Treat. 2012;133(2):713-723.
doi pubmed - Riedlinger GM, Joshi S, Hirshfield KM, Barnard N, Ganesan S. Targetable alterations in invasive pleomorphic lobular carcinoma of the breast. Breast Cancer Res. 2021;23(1):7.
doi pubmed - Kada Mohammed S, Billa O, Ladoire S, Jankowski C, Desmoulins I, Poillot ML, Coutant C, et al. HER2-positive invasive lobular carcinoma: a rare breast cancer which may not necessarily require anti-HER2 therapy. A population-based study. Breast Cancer. 2023;30(3):343-353.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.