| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 000, Number 000, July 2025, pages 000-000
Mechanism of Action of Resveratrol Affecting the Biological Function of Breast Cancer Through the Glycolytic Pathway
Yu Gaoa, Yao Yao Wangb, Bao Di Wangb, Qun Ying Hub, Ji Rui Jiangc, Bo Fengd, Xiu Li Gaoe, Li Kun Liue, Wen Bin Zhue, f, Li Ling Yuee, f
aPhase I Clinical Research Center, The Third Affiliated Hospital of Qiqihar Medical University, Qiqihar, Heilongjiang, China
bDepartment of Medical Technology, Qiqihar Medical University, Qiqihar, Heilongjiang, China
cDepartment of Basic Medicine, Qiqihar Medical University, Qiqihar, Heilongjiang, China
dDean’s Office, Qiqihar Medical University, Qiqihar, Heilongjiang, China
eResearch Institute of Medicine and Pharmacy, Qiqihar Medical University, Qiqihar, Heilongjiang, China
fCorresponding Authors: Li Ling Yue, Qiqihar Medical University, Qiqihar 161000, Heilongjiang, China; Wen Bin Zhu, Qiqihar Medical University, Qiqihar 161000, Heilongjiang, China
Manuscript submitted April 5, 2025, accepted June 11, 2025, published online July 8, 2025
Short title: Resveratrol Affects Breast Cancer Glycolysis
doi: https://doi.org/10.14740/wjon2586
| Abstract | ▴Top |
Background: Phosphoglycerate kinase 1 (PGK1) plays a crucial role in the glycolytic pathway and its overexpression has a negative impact on tumor development and prognosis. Resveratrol, a natural polyphenolic compound, has gained significant attention in recent years due to its anti-inflammatory, antioxidant, and anti-tumor properties. However, the mechanism by which resveratrol inhibits breast cancer growth, invasion, and metastasis through the PGK1 glycolytic pathway is still not fully understood.
Methods: We used the Gene Expression Profiling Interactive Analysis (GEPIA) and the Human Protein Atlas database to analyze the expression levels of glycolytic enzymes in different breast tissues and their correlation with the prognosis of breast cancer patients. The effect of resveratrol on the biological functions of breast cancer was studied through wound healing experiments and Transwell migration and invasion experiments. Reverse transcription quantitative polymerase chain reaction (RT-qPCR), Western blot, and in vivo mouse tumorigenesis experiments were used to explore the possible molecular mechanism of resveratrol inhibiting the occurrence and development of breast cancer.
Results: Resveratrol exerted oncogenic effects both in vivo and in vitro. In our study, we provided additional evidence to support the role of resveratrol in breast cancer treatment. Specifically, we found that resveratrol effectively reduced the expression of PGK1 in BT-549 cells. This reduction is achieved by regulating an important transcription factor c-Myc. As a result, the cellular glycolytic pathway is blocked, leading to the inhibition of malignant biological behavior in breast cancer cells.
Conclusion: Our findings suggest that targeting the PGK1 glycolytic pathway could be a promising approach for resveratrol-based treatment of breast cancer.
Keywords: Resveratrol; Breast cancer; Glycolysis; Invasion; Apoptosis
| Introduction | ▴Top |
Breast cancer is a prevalent malignant disease among women, surpassing lung cancer in 2020 with a high incidence of 2.26 million cases and becoming the most common cancer worldwide. Surprisingly, the incidence rate of breast cancer among Chinese women of childbearing age accounts for 25% and represents 10% of all malignant tumors. This rate shows an increasing trend every year [1]. However, metastatic progression substantially reduces survival rates. Although therapeutic advances have reduced mortality, breast cancer remains the leading cause of cancer-related death in women globally.
Resveratrol is a natural polyphenolic compound that can be found in various sources including blueberries, mulberries, Polygonum multiflorum, and cassia seeds [2]. It possesses numerous pharmacological activities such as anti-inflammatory, antioxidant, anti-tumor, metabolism regulation, and immune system modulation. Resveratrol has been shown to inhibit the growth of various types of tumors including colon cancer, pancreatic cancer, ovarian cancer, and breast cancer [3]. Resveratrol’s anti-tumor properties have garnered significant research attention. Nevertheless, its mechanism of action remains incompletely characterized and warrants further investigation. Studies demonstrate that resveratrol suppresses preneoplastic lesion formation and metastasis by modulating cancer cell proliferation, apoptosis induction, and angiogenic pathways [4]. It exerts its anti-tumor activity by directly targeting relevant factors. These findings serve as a valuable reference for the development of new anti-tumor drugs and demonstrate significant inhibitory effects on various types of tumors [5]. Additionally, resveratrol blocks the cell cycle by activating the oncogenes p53 and suppressor gene PTEN, while also down-regulating phosphatidylinositol 3-kinase (PI3K). Ultimately, this leads to the induction of apoptosis in cancer cells [6]. Therefore, the chemopreventive potential of resveratrol in human oncology has gained substantial understanding. Evidence has been accumulated from in vitro models, animal cancer research, and epidemiological observations to support their abilities.
Phosphoglycerate kinase 1 (PGK1) is an essential enzyme in glycolysis, serving as both a metabolic enzyme and a protein kinase. It plays a crucial role in tumor growth, invasion, and metastasis by phosphorylating key substrates. As a metabolic enzyme, the primary function of PGK1 is to participate in the glycolysis reaction [7, 8]. PGK1 is a crucial molecular target in tumor therapy and has gained significant attention in recent years [9]. Recent studies have demonstrated that PGK1 plays a role in promoting breast cancer cell growth and lactate production, making it a potential target gene for breast cancer treatment [10]. Moreover, downregulation of PGK1 has been shown to significantly inhibit the invasive ability of breast cancer cells, reverse the process of epithelial-mesenchymal transition, and enhance the Warburg effect [11]. PGK1 is highly expressed in metastatic and invasive ductal breast cancer cells. It is significantly correlated with advanced tumor stage, suggesting its close association with breast cancer onset, progression, and metastasis [12]. In conclusion, reducing PGK1 expression offers a promising strategy to inhibit tumor growth in the future. Therefore, PGK1 can serve as an important biomarker for targeted tumor therapy.
| Materials and Methods | ▴Top |
Reagents
Resveratrol (≥ 99%, high-pressure liquid chromatography (HPLC)) was obtained from the National Institutes for Food and Drug Contro (Beijing, China). RPMI 1640 medium was purchased from Biosharp (Anhui, China). Hexokinase (HK), pyruvate kinase (PK), and lactate dehydrogenase (LDH) activity assay reagents were purchased from Solarbio Company (Beijing, China). PGK1 enzyme-linked immunosorbent assay (ELISA) reagents were purchased from Jianglai Biological (Shanghai, China). Muse caspase-3/7 kit was purchased from Luminex Company (Shanghai, China). Enhanced ATP detection reagent was purchased from Beyotime Biotechnology (Shanghai, China). PGK1, c-Myc, BAX, and matrix metalloproteinase (MMP)2/9 antibodies were purchased from Cell Signaling Technology (Boston, USA). Antibodies to caspase-3 and caspase-7 were purchased from Wanlei Biotechnology (Shenyang, China).
Bioinformatics analysis
The Gene Expression Profiling Interactive Analysis (GEPIA) [13] is able to predict PGK1 expression in breast cancer samples, in order to explore the expression level of PGK1 in breast cancer and its relationship with patients’ prognostic survival. The Human Protein Atlas [14] was obtained by analyzing the correlation between the expression level of human gene mRNA in tumor tissue and the prognostic data of cancer patients, the immunohistochemical (IHC) map of cancer tissue, and the relationship between the expression of specific genes and the occurrence and development of specific tumors.
Molecular docking predicts the binding energy of resveratrol and PGK1
Molecular docking of resveratrol with PGK1 was performed by AutoDock software to detect the docking binding energy. The chemical structure of the ligand was downloaded from the ZINC database [15], and the Auto Dock Tools tool was used to set up a Grid Box centered on the ligand, and good docking active sites were obtained in the Autogrid module.
Cell culture
Human breast cancer cell BT-549 is from the Shanghai Cell Bank of the Chinese Academy of Medical Sciences. It is regularly cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS), in which 57.5 µL/100 mL insulin was added, and cultured at 37 °C with 5% CO2 for 1 - 3 days for passage treatment.
Cell proliferation assay
The MTT assay was employed to investigate the impact of varying concentrations of resveratrol on the proliferation of BT-549 breast cancer cells. Briefly, resveratrol final concentrations of 2, 4, 8, 16, 32, 64, and 128 µg/mL were used in sterile 96-well plates inoculated with breast cancer BT-549 cells (6 × 103 cells/well) for 24 h. For the experiment, we utilized 180 µL of serum-free cell culture medium and added 20 µL of 5 mg/mL MTT solution (Beyotime, China) to continue incubation for 4 h. Afterwards, 150 µL of DMSO (Sigma-Aldrich, USA) was added to each well, and the crystals were dissolved by shaking on a shaker for 10 min. The absorbance value was measured at 570 nm using an enzyme-labeling measuring, and the cell half inhibition rate (IC50) was calculated.
HK, PK, LDH, and PGK1 enzyme activity assay
The activities of HK and PK enzymes were measured using a UV spectrophotometer assay, while the LDH enzyme activity was measured using the enzyme-labeling measuring instrument. Firstly, breast cancer BT-549 1 × 104 cells were taken for the experiment. Secondly, for HK and PK enzyme activity assay, the cell supernatant and the assay reagent were mixed according to the instructions, the absorbance value was detected at 340 nm by a UV spectrophotometer, and the enzyme activity was calculated. For LDH enzyme activity, 100 µL of standard solution was taken for concentration dilution, a standard curve was made, the test solution was prepared according to the steps in the instruction manual, and the absorbance value was detected at 570 nm by an enzyme meter. After that, PGK1 enzyme activity was detected using ELISA kit, and the OD values of each experimental group were detected at 450 nm with enzyme-labeling measuring.
ATP content assay
When the BT-549 cell fusion reached 80-90% in a sterile 12-well plate, 150 µL ATP lysis buffer was added to each well and centrifuged at 4 °C 12,000 rpm for 5 min, and the supernatant was taken for experiments, and the luminometer program of the enzyme marker was selected to determine the relative light unit (RLU) value of the sample.
Cell migration and invasion assay
The inhibitory effect of resveratrol on the migration and invasion ability of breast cancer BT-549 cells was detected by wound healing, transmembrane migration, and invasion assays. In the wound healing experiment, BT-549 cells (1 × 105/well) were cultured in a six-well plate for 24 h and allowed to reach 70% and 80% cell fusion, then the tip of a sterile 200 µL gun was used to scratch the cells perpendicular to the plane of the six-well plate, and then the cells were washed with phosphate-buffered saline (PBS) for three times and the cell migration was monitored under an inverted microscope for more than 24 h. Finally, the ImageJ software was used for analysis.
For Transwell invasion, a Transwell chamber (Corning, USA) with an 8 µm hole polycarbonate filter was used. Briefly, 50 mg/L of Materiel (BD, Biosciences, USA) was diluted with sterile PBS in a 1:8 ratio, and 50 µL was added to the upper chamber, which was allowed to solidify at 37 °C for 2 h. After that, 100 µL of medium containing 1% FBS was added to the upper chamber, and the incubation was performed at 37 °C for about 30 min. Then, the cells were incubated with resveratrol (20, 40, and 60 µg/mL) for 24 h and digested with RPMI-1640 serum-free medium. A cell suspension of 100 µL (medium containing 1% FBS) containing 2.5 × 104/mL invasive cells was inoculated into the upper chamber coated with 50 µL of matrigel, and 600 µL of serum-containing medium was added to the lower chamber for 24 h of incubation, and non-migratory cells at the top of the upper chamber were removed with a cotton swab. The cells were fixed with 4% paraformaldehyde (Beyotime, China) and stained with 0.1% crystal violet for 1 h.
For migration experiments, BT-549 cells treated with different concentrations of resveratrol were inoculated into top chambers without matrigel coating, and other steps were the same as for invasion experiments.
Apoptosis detection
BT-549 cells (1 × 105 - 5 × 106 cells/mL) were suspended in 1× BA assay buffer and 50 µL was added to each experimental tube. Positive and negative control staining was performed, followed by the addition of 5 µL of Muse caspase-3/7 reagent working solution to each tube. The tubes were then incubated at 37 °C in a 5% CO2 incubator for 30 min. Subsequently, 150 µL of Muse caspase7-AAD working solution was added to each tube and mixed. The tubes were incubated at room temperature for 5 min, avoiding light. Apoptosis was detected using a Muse flow cytometer (Merck-Millipore, Germany).
Western blotting analysis
BT-549 cells were treated with resveratrol (20, 40, and 60 µg/mL) for 24 h, the cells were collected and lysed, and then quantified by bicinchoninic acid (BCA) protein assay kit (Beyotime, China). The protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride (PVDF) membrane and sealed in 5% skimmed milk powder solution for 1 h. After incubating the blotting membrane with the corresponding primary antibody, and then incubating with the second antibody bound to horseradish peroxidase, the target protein band was detected by enhanced chemiluminescence under the scanning MP imaging system (Bio-Rad, USA).
Real-time quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from BT-549 cells by applying TRIzol reagent (Invitgen, USA). cDNA was synthesized using Prime SCRIPT RT kit (Takara, Japan) in order to detect the gene expression level. qRT-PCR reaction was performed using SYBR Green (Takara, Japan). With β-actin gene as an internal reference, qRT-PCR was performed to detect the expression of PGK1 and c-Myc genes, and the relative expression was calculated by 2-ΔΔct: PGK1 (F, 5'-GTGAAGATTACCTTGCCTGTT-3'; R, 5'-GCTTCCCATTCAAATACCC-3'), c-Myc (F, 5'-CCGCCTGCGATGATTTATAC-3'; R, 5'-CAGCCGAGCACTCTAGCTCT-3'), β-actin (F, 5'-GAGCGGGAAATCGTGCGTGACAT-3'; R, 5'-CAGGAAGGAAGGCTGGAAGAGTG-3').
Tumor growth and metastasis in mice with breast cancer
The 4T1 murine mammary carcinoma cells were purchased from the Institute of Basic Medical Science, Chinese Academy of Medical Sciences (Beijing, China). Mouse breast cancer 4T1 cells (2 × 106 cells/0.2 mL) were subcutaneously injected into the right axilla of female BALB/c mice to establish a 4T1 tumor mouse (age: 4 - 6 weeks; body weight: 18 - 22 g, n = 6, SPF grade) model. The mice were randomly divided into five groups, and when the tumor volume was about 300 - 400 mm3, each group was intraperitoneally injected with 0.2 mL of normal saline 0.2 mL/kg, cyclophosphamide (CTX) solution 2.5 mg/kg, and resveratrol solution (20, 50, and 100 mg/kg). After 21 days of continuous administration, mice were sacrificed and histologically examined by hematoxylin and eosin (H&E) staining, and the expression of PGK1, proliferating cell nuclear antigen (PCNA), and MMP-2/9 protein in tumor tissue was detected by IHC.
Statistical analysis
The experimental data were completed in three sessions and statistical differences were expressed as mean ± standard deviation. Comparisons between groups were made using analysis of variance and t-tests, with differences considered statistically significant at P < 0.05 and significant at P < 0.01.
Ethical considerations
This study was approved by the Ethics Committee of Qiqihar Medical University. (Ethical code QMU-AECC-2022-143). The study was conducted in accordance with all applicable institutional ethical guidelines for the care, welfare and use of animals.
| Results | ▴Top |
PGK1 could be a better glycolytic target for breast cancer
Abnormal expression of metabolic enzymes in glycolysis is one of the main causes of Warburg effect, which is one of the hallmarks of cancer. Thus, we firstly analyzed the expression levels of different glycolytic enzymes between different breast cancer tissues and normal tissues through GEPIA database (Fig. 1a). The results indicated that phosphofructokinase (PFKP) and lactate dehydrogenase A (LDHA) showed no significance between breast cancer and normal epithelial tissues, and hexokinase 2 (HK2) only showed higher expression levels in human epidermal growth factor receptor 2 (HER2) breast cancers. However, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and PGK1 were highly expressed in Basal-like, HER2, and luminal-B breast cancers and M-type pyruvate kinase (PKM) could be more effective targets for most breast cancers. In addition, the IHC results of GAPDH, PGK1, and PKM from Human Protein Atlas database indicated that the ratio of breast cancer tissues performing “medium/high” staining was higher than that of normal tissues, which mainly performed “not detected/low” staining results (Fig. 1b-d), further proving our prediction. To further elucidate the research significance of above targets, we analyzed their correlation with the prognosis of breast cancer patients through GEPIA and the Human Protein Atlas database (Fig. 1e-g). The analysis results of these two databases showed that there was a significant correlation between the poor prognosis and higher PGK1 expression level, but not that of GAPDH. About PKM, only the Human Protein Atlas database suggested a negative correlation between PKM expression and patients’ prognosis. The results suggested that PGK1 and PKM might serve as a promising target for breast cancer, especially for PGK1.
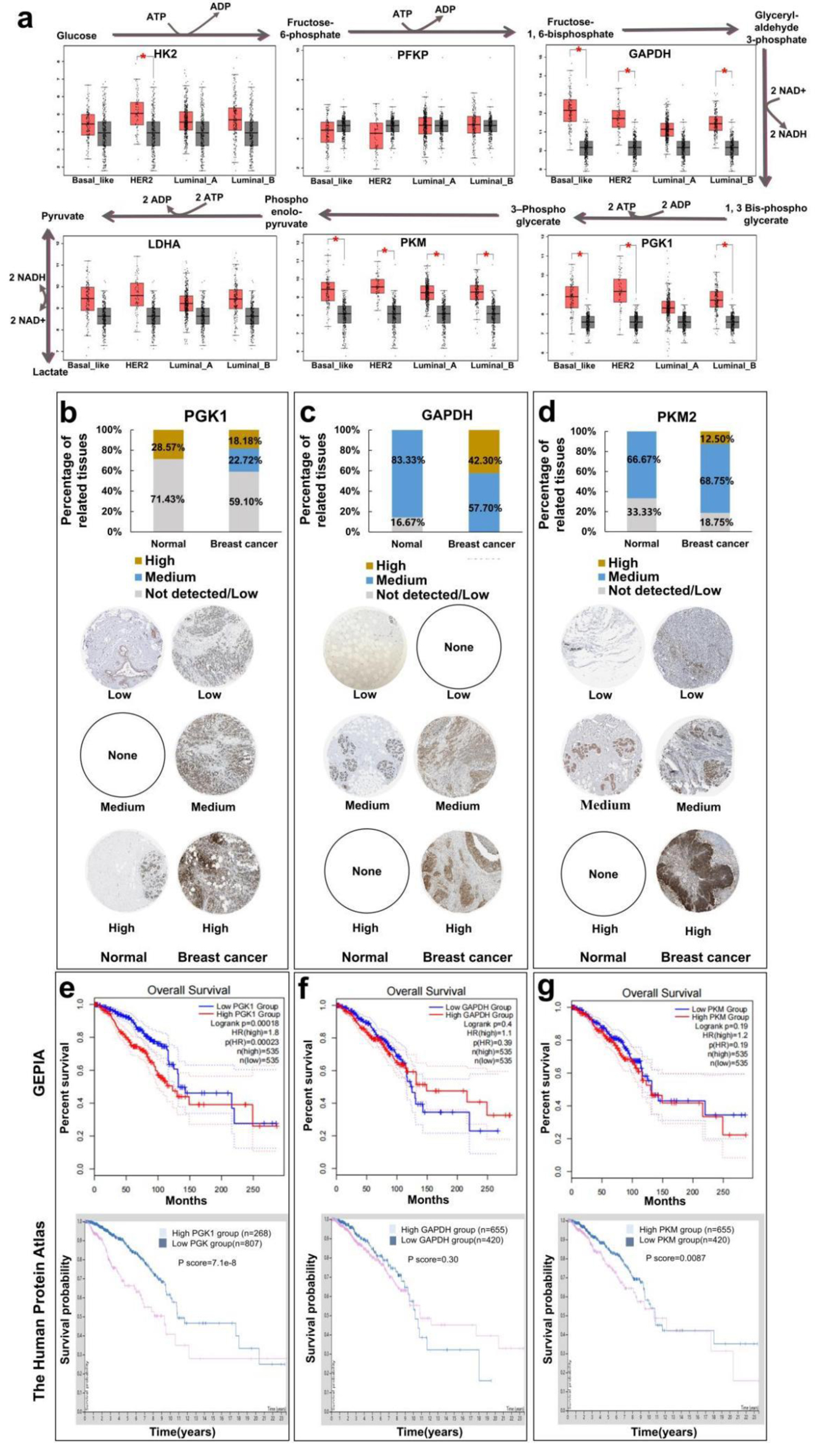 Click for large image | Figure 1. Analysis of survival and prognosis of glycolytic enzymes in different breast cancers by database. (a) Relationship between expression levels of glycolytic metabolic enzyme (HK2, PFKP, GAPDH, LDHA, PKM, and PGK1) of breast cancer. (b) Human Protein Atlas analyzes the immunohistochemical expression of PGK1 enzymes in breast cancer. (c) Immunohistochemical expression of GAPDH enzymes in breast cancer. (d) Immunohistochemical expression of PKM2 enzymes in breast cancer. (e) GEPIA and Human Protein Atlas analysis of relationship between expression levels of glycolytic metabolic enzyme PGK1 and overall survival of breast cancer patients. (f) Relationship between expression levels of glycolytic metabolic enzyme GAPDH and overall survival of breast cancer patients. (g) Relationship between expression levels of glycolytic metabolic enzyme PKM2 and overall survival of breast cancer patients. GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GEPIA: The Gene Expression Profiling Interactive Analysis; HK2: hexokinase 2; LDHA: lactate dehydrogenase A; PFKP: phosphofructokinase; PGK1: phosphoglycerate kinase 1; PKM: M-type pyruvate kinase. |
Resveratrol inhibits PGK1 and glycolysis in breast cancer
As a classical antineoplastic drug, resveratrol has been reported to inhibit some of glycolysis enzymes, including PFKP, HK2, and PKM. However, to the best of our knowledge, effects of resveratrol on PGK1 remain unknown. Thus, we detected effects of resveratrol on PGK1, from both the expression and activity points of view. qRT-PCR and Western blot results suggested that resveratrol could inhibit mRNA and protein expression in a dose-dependent manner (Fig. 2a, b). In addition, the molecular docking results indicated that resveratrol could also combine with multiple amino acids of PGK1 automatically with a binding energy less than -5.0 kcal/mol. Among the combined amino acids, 291 and 292 were located in the same active pocket with amino acids from 273 to 276 (the substrate binding sites of PGK1), with a minimum distance of 8.3 Å (Fig. 2c). The binding site of resveratrol and PGK1 was located in overlapping active pockets, which formed a similar force with the key residues of the active site. This indicated that in addition to PGK1 expression, resveratrol might also competitively inhibit PGK1 activity through their direct combination [16]. Consistent with the above results, resveratrol inhibited the catalytic activity of PGK1 in a dose-dependent manner (Fig. 2d). As PGK1 is the key rate-limited enzyme of glycolysis, we further detected the effects of PGK1 on glycolysis. According to Figure 2e, f, resveratrol can obviously inhibit catalytic activities of PK and LDH, which were located downstream of PGK1. What’s more, resveratrol can inhibit ATP contents in glycolysis (Fig. 2g). All the above results proved the inhibitory effects of resveratrol on PGK1 and glycolysis.
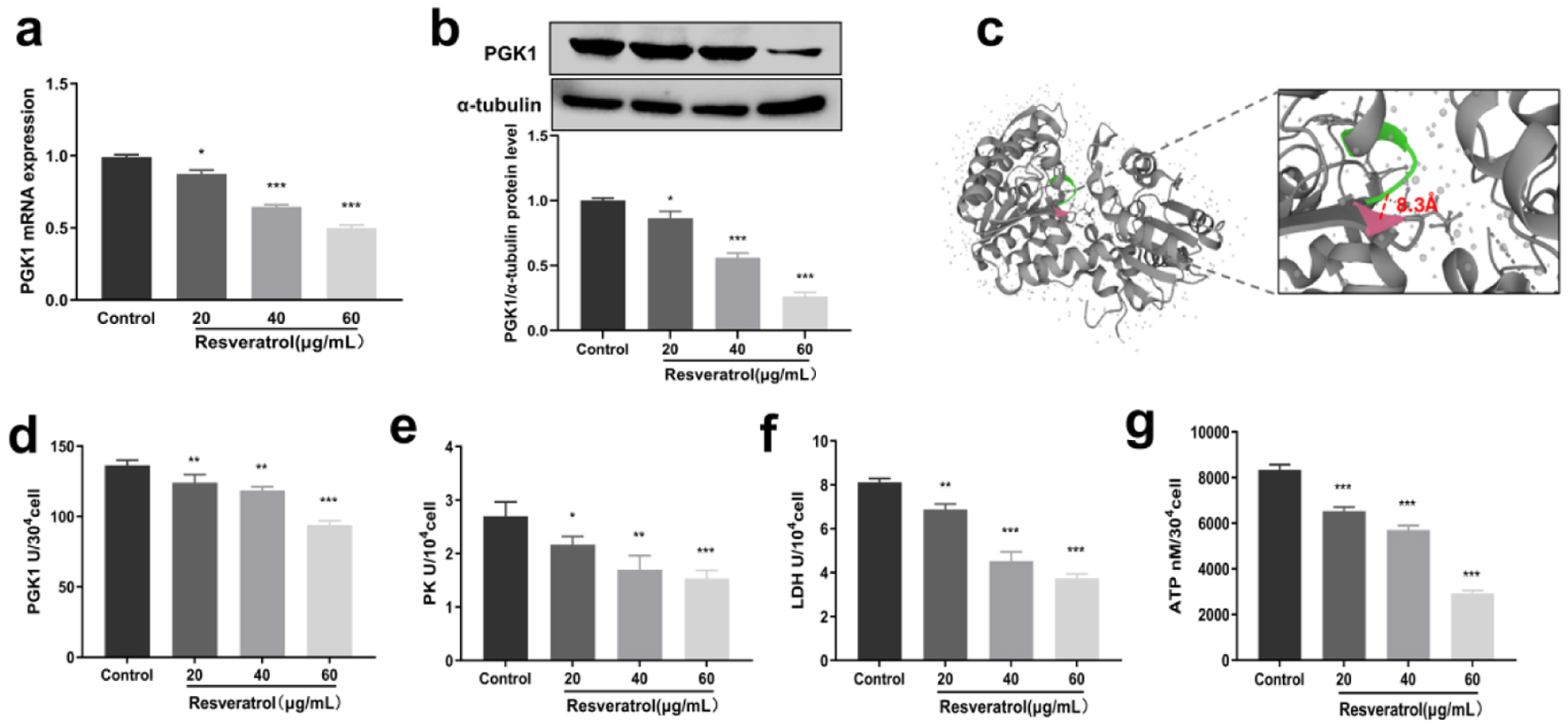 Click for large image | Figure 2. Inhibitory effect of resveratrol on PGK1 and glycolytic enzymes. (a) Inhibition of PGK1 mRNA expression by resveratrol. (b) Inhibition of PGK1 protein expression by resveratrol, and α-tubulin was used as loading control. (c) Molecular docking of resveratrol with PGK1. (d) Inhibitory effect of resveratrol on PGK1 enzyme activity. (e) Inhibitory effect of resveratrol on PK enzyme activity. (f) Inhibitory effect of resveratrol on LDH enzyme activity. (g) Inhibitory effect of resveratrol on ATP content. LDH: lactate dehydrogenase; PGK1: phosphoglycerate kinase 1; PK: pyruvate kinase. |
Resveratrol inhibits PGK1-related c-Myc/PI3K/AKT axis
In order to gain a thorough understanding of the potential mechanism by which resveratrol inhibits PGK1 expression, we assessed the effects of resveratrol on c-Myc, which is a well-known transcription factor that can regulate PGK1 expression [17]. Our results revealed that resveratrol reduced c-Myc mRNA and protein expression levels (Fig. 3a, b). Moreover, resveratrol showed synergistic inhibitory effects on the expression level of c-Myc and PGK1, indicating that resveratrol inhibits PGK1 partially through the inhibition of c-Myc (Fig. 3c, d). As a well-known oncogene, PGK1 can active PI3K/AKT signaling pathway [18], which leads to the phosphorylation and inactivation of GSK-3β and prevents GSK-3β from binding to β-catenin. Consequently, β-catenin accumulates and translocates into the nucleus, inducing transcription of target genes [19]. In addition, PGK1 can also contribute to the stabilization of GSK-3β, and then promotes β-catenin expression and maintains the stemness of breast cancer cells [20]. Our results demonstrated that resveratrol significantly inhibited the phosphorylation of PI3K and AKT, and the expression of GSK-3β and β-catenin (Fig. 3e, f). These findings suggested that resveratrol could exert anti-breast cancer effects by regulating PGK1 related c-Myc/PI3K/AKT axis.
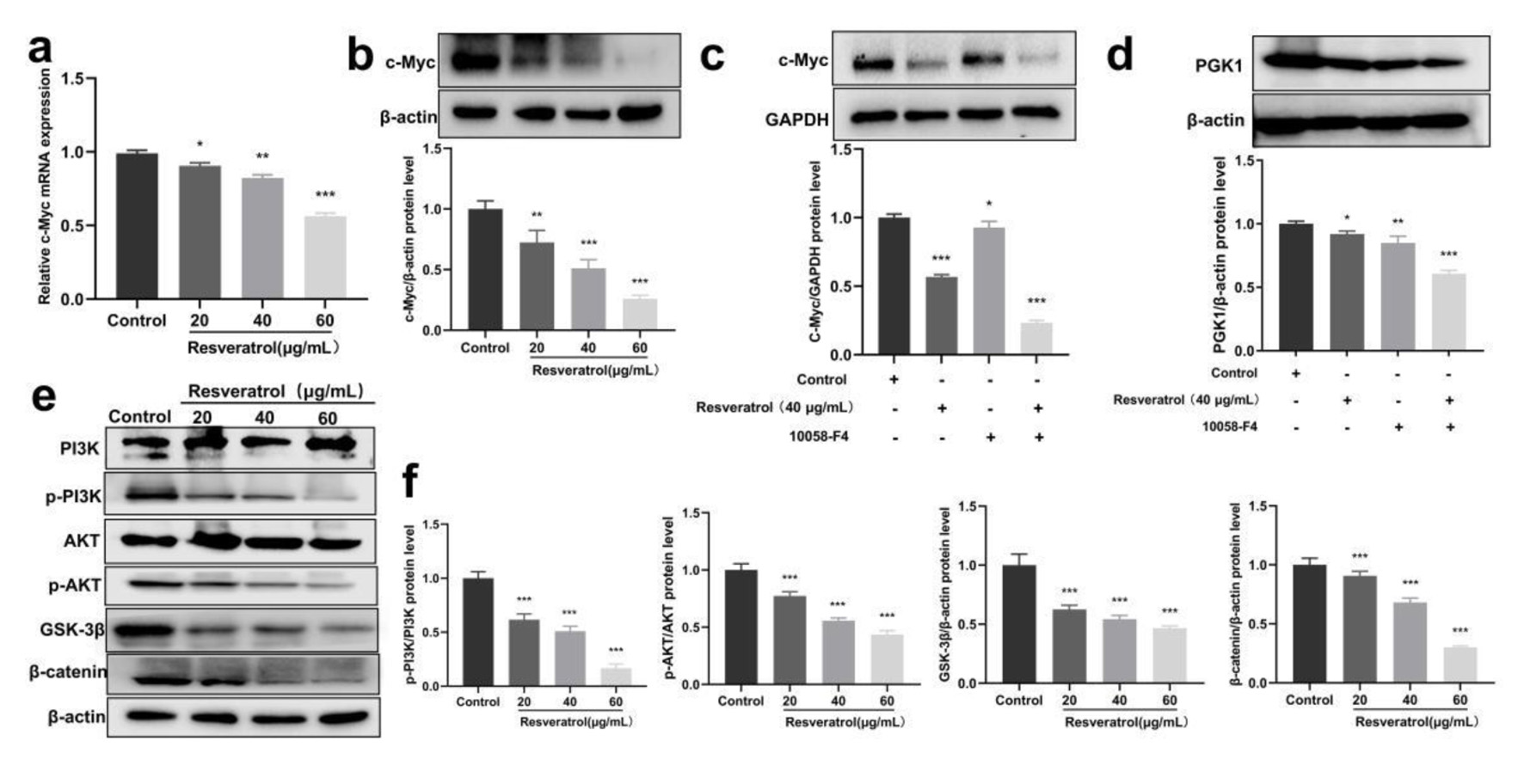 Click for large image | Figure 3. Resveratrol inhibits PGK1-related c-Myc/PI3K/AKT axis. (a) Resveratrol (20, 40, and 60 µg/mL) on the expression of c-Myc mRNA in BT-549 cells. (b) Resveratrol (20, 40, and 60 µg/mL) on the expression of c-Myc protein in BT-549 cells. (c) c-Myc protein expression induced by resveratrol (40 µg/mL) and 10058-F4 (90 µM) alone and in combination. (d) PGK1 protein expression induced by resveratrol (40 µg/mL) and 10058-F4 (90 µM) alone and in combination. (e) Western blot detection for phosphorylation-related proteins in the PI3K/AKT/GSK-3β/β-catenin pathway. (f) Quantification of Figure 3e. *P < 0.05, **P < 0.01, ***P < 0.001. PGK1: phosphoglycerate kinase 1; PI3K: phosphatidylinositol 3-kinase. |
Resveratrol inhibits cell proliferation and induces apoptosis in breast cancer
The glycolytic pathway is one of the main hallmarks of cancer, which provides rapid ATP supplementation for cell proliferation. In addition, PGK1 is also a kind of oncogene which involves the initiation and development of several cancers. Thus, based on the inhibition effects on PGK1, we discussed anti-tumor effects of resveratrol in BT-549 cells, which has not been reported before. MTT methods revealed that resveratrol could inhibit BT-549 cell viability in a dose- and time-dependent manner, with an IC50 of 40.55 ± 3.39 µg/mL at 24 h (Fig. 4a). In addition, resveratrol also reduced the expression level of proliferation marker PCNA (Fig. 4b), which confirmed the inhibitory effect of resveratrol on cell proliferation together with MTT results. Furthermore, our flow cytometry experiments demonstrated that resveratrol increased the proportion of apoptotic cells in a dose-dependent manner (Fig. 4c, d). Moreover, apoptosis-related proteins such as the Bax/Bcl-2 ratio, cleaved caspase-3, and cleaved caspase-7 were also promoted by resveratrol (Fig. 4e, f). All the results above revealed that resveratrol could inhibit cell proliferation and induce apoptosis in breast cancer.
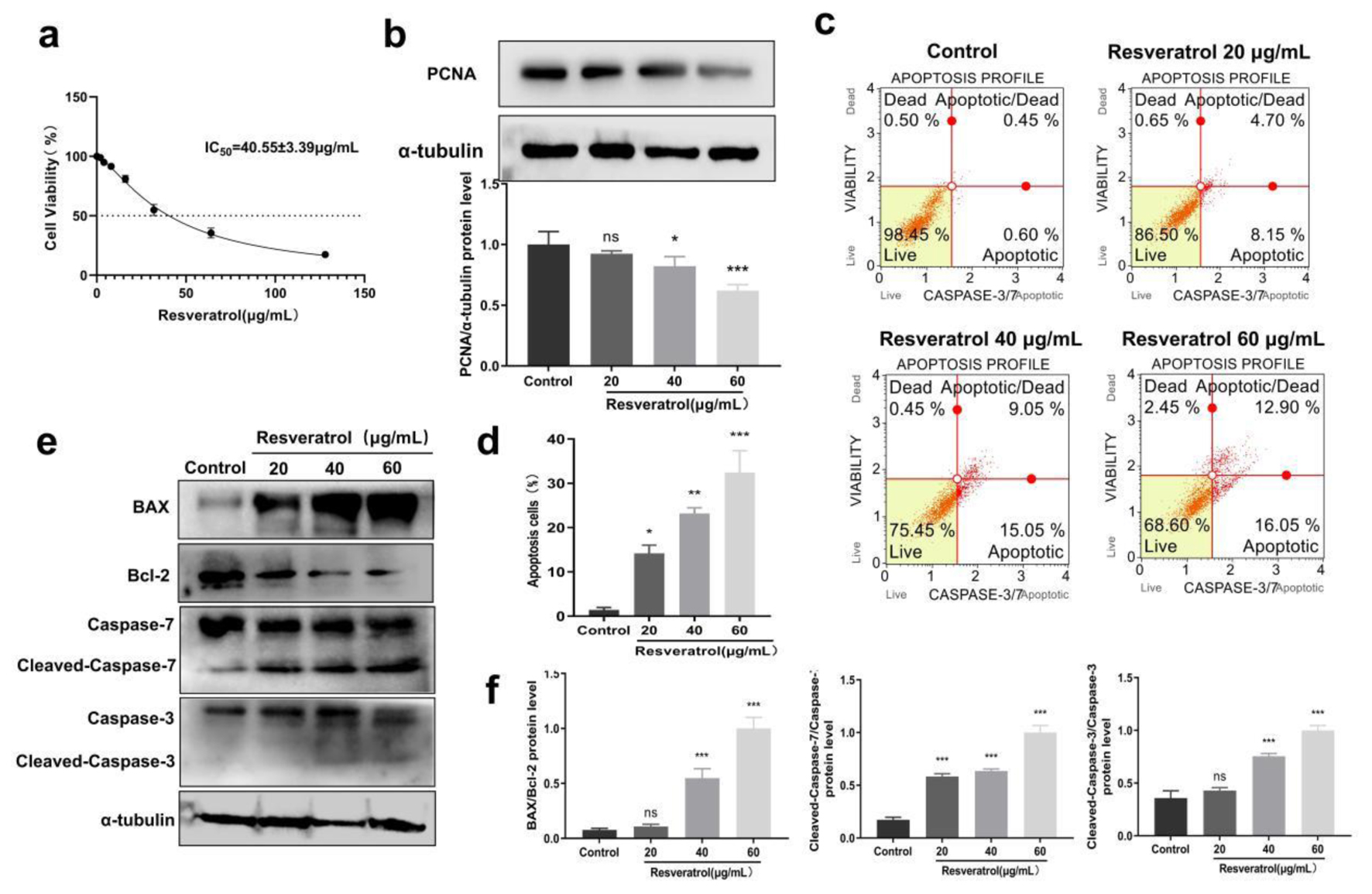 Click for large image | Figure 4. Resveratrol inhibits cell proliferation and induces apoptosis in breast cancer. (a) Inhibitory effects of resveratrol on BT-549 cells detected by MTT methods, and IC50 values were calculated through Graphpad Prism. (b) Resveratrol (20, 40, and 60 µg/mL) on PCNA protein expression in BT-549 cells. (c) Resveratrol (20, 40, and 60 µg/mL) on apoptosis rate of BT-549 cells. (d) Quantification of Figure 4c. (e) Resveratrol (20, 40, and 60 µg/mL) on the protein expression of BAX, Bcl-2, caspase-3/7 in BT-549 cells, and α-tubulin was used as loading control. (f) Quantification of Figure 4e. *P < 0.05, **P < 0.01, ***P < 0.001. PCNA: proliferating cell nuclear antigen. |
Resveratrol inhibits cell migration and invasion in breast cancer
It has been reported that targeting PGK1-mediated Warburg effect could suppress breast tumor metastasis [21]. Although resveratrol has been reported to inhibit migration and metastasis of some breast cancer cells including MDA-MB-231 [22], related effects on BT-549 cells remain unknown. Thus, we detected the effects of resveratrol on BT-549 cell migration and invasion. The scratch-wound experiment demonstrated that after being scratched for 24 h, the wound healing rate of BT-549 cells was 59.19±3.585%, indicating its highly metastatic potential. However, treatment with resveratrol significantly inhibited the wound healing rate of BT-549 cells (Fig. 5a, b). In addition, the Transwell experiment without the matrix also confirmed the inhibitory effects of resveratrol on BT-549 cells (Fig. 5c, d). The number of invasion cells decreased from 198 ± 6.07 (control group) to 98.33 ± 5.074 (20 µg/mL), 73 ± 6.245 (40 µg/mL), and 44.62 ± 4.163 (60 µg/mL), respectively (Fig. 5e, f). What’s more, Western blot experiments revealed that resveratrol dose-dependently inhibited the expression levels of migratory invasion markers MMP2 and MMP9 (Fig. 5g, h). Collectively, these findings provided evidence for the anti-breast cancer effects of resveratrol.
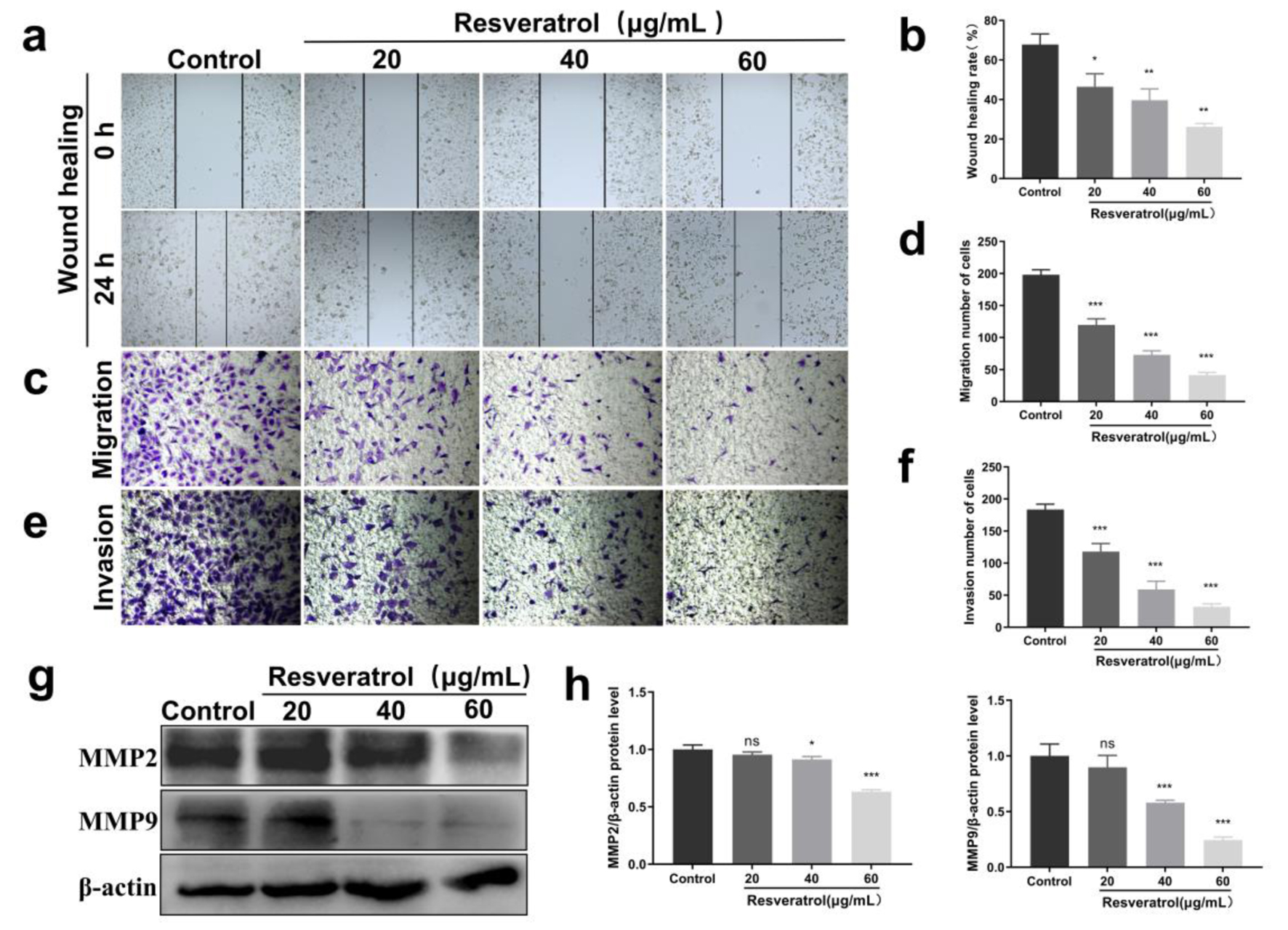 Click for large image | Figure 5. Effects of resveratrol on migration and invasion of BT-549 cells. (a) Scratch assay to detect the effect of resveratrol (20, 40, and 60 µg/mL) on the wound scratching ability of BT-549 cells. (b) Quantification of Figure 5a. (c) Transwell assay to detect the effect of resveratrol (20, 40, and 60 µg/mL) on the migration ability of BT-549 cells. (d) Quantitative results of Figure 5c. (e) Transwell assay to detect the effect of resveratrol (20, 40, and 60 µg/mL) on the invasive metastatic ability of BT-549 cells. (f) Quantitative results of Figure 5e. (g) Resveratrol (20, 40, and 60 µg/mL) on the expression of MMP2 and MMP9 proteins in BT-549 cells, and β-actin was used as loading control. (h) Quantitative results of Figure 5g. *P < 0.05, **P < 0.01, ***P < 0.001. MMP: matrix metalloproteinase. |
In vivo anti-tumor effects of resveratrol on breast cancer
Finally, we investigated the in vivo anti-mammary effects of resveratrol. We conducted experiments using breast cancer cells to induce mouse tumors (model group), which were then treated with different doses of resveratrol (treatment group). We used the well-known anti-tumor drug CTX as a positive control. The results from H&E staining revealed that the tumor tissue in the model group exhibited vigorous growth, with a large number of tightly packed cancer cells showing irregular morphology and darker nuclear staining color. However, after treatment with resveratrol at concentrations of 20, 50, and 100 mg/kg, the dense structure of the tumor was damaged, leading to a gradual restriction of tumor tissue growth. The tumor cells also exhibited varying degrees of shrinkage and rounding, and the arrangement of the tumor cells became progressively sparse. The CTX group showed the most significant inhibition of tumor growth in the breast cancer hormone mice, with a large area of necrosis and dissolution observed in the tissue (Fig. 6a). In addition, IHC staining results demonstrated that resveratrol could inhibit the positive staining of proteins including PGK1, PCNA, and MMP9 (Fig. 6b, c). Notably, the above effects of 100 mg/kg resveratrol were similar to that of the positive control drug CTX. Finally, we observed that resveratrol could significantly inhibit the tumor volume (Fig. 6d, e) and tumor weight (Fig. 6f) but did not affect the body weight (Fig. 6g) of the tumor-bearing mice.
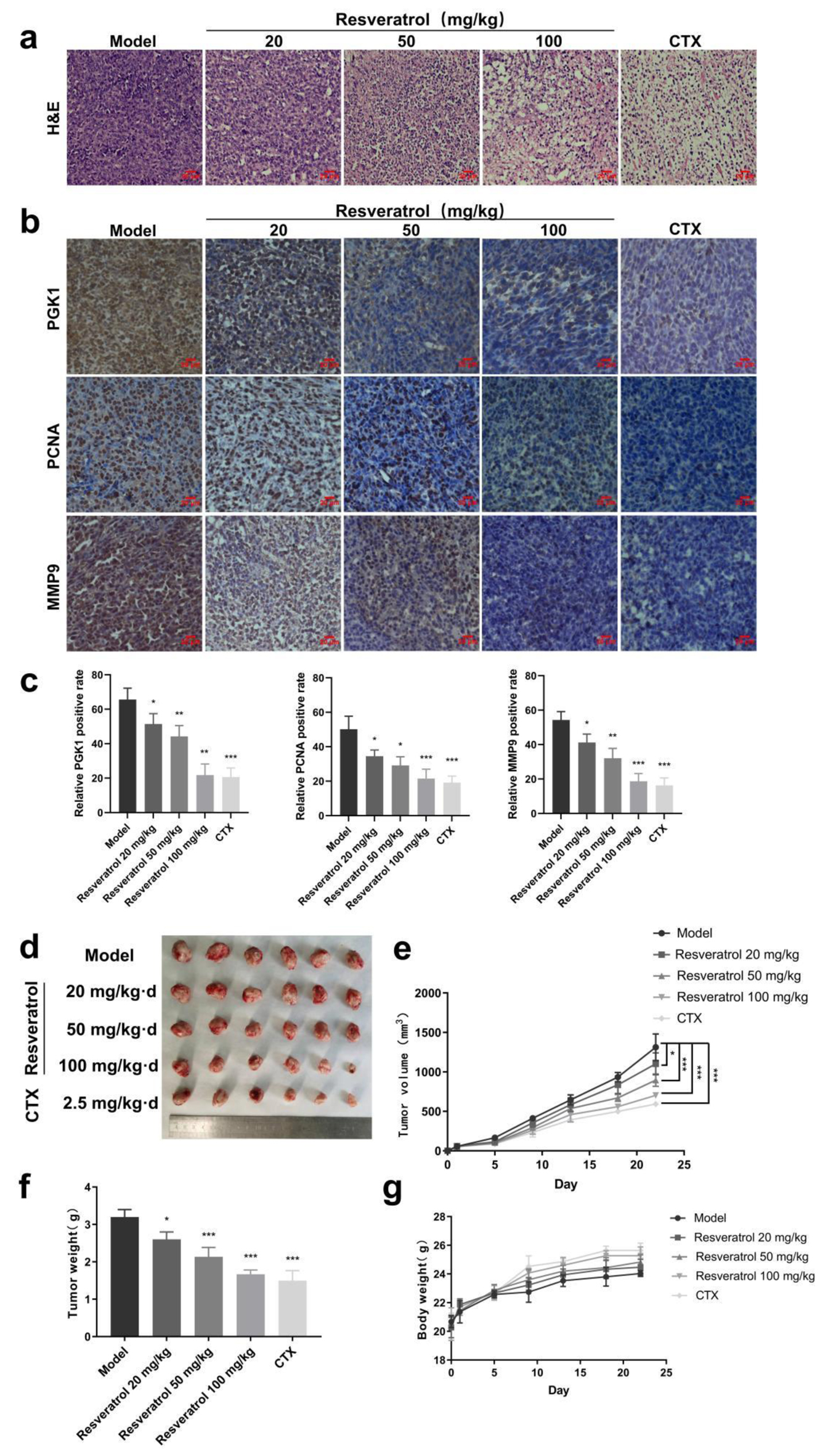 Click for large image | Figure 6. Anti-tumor effect of resveratrol in vivo. (a) Pathological examination of tumor tissue in vivo detected by H&E staining. (b) IHC staining to detect the differences in the expression of relevant proteins in tumor tissues. (c) Quantitative results of Figure 6b. (d) Effect of resveratrol (20, 50, and 100 mg/kg) and CTX on the tumor volumes of loaded mise. (e) Quantitative results of Figure 6d. (f) Effect of resveratrol (20, 50, and 100 mg/kg) and CTX on tumor weight in hormonal mice. (g) Effects of resveratrol (20, 50, and 100 mg/kg) and CTX on the body weights of the mise. *P < 0.05, **P < 0.01, ***P < 0.001. CTX: cyclophosphamide; H&E: hematoxylin and eosin; IHC: immunohistochemistry. |
| Discussion | ▴Top |
Breast cancer, a malignant epithelial neoplasm arising from the mammary glandular tissue, demonstrates persistently rising global incidence based on contemporary epidemiological surveillance. Notably, breast cancer superseded lung cancer as the most prevalent malignancy worldwide in 2020, constituting the principal oncological burden among female populations [23]. In China, age-standardized incidence rates rose by 3.1% annually during 2000 - 2018, exceeding global averages [24]. The current dominant approach in Western medicine for treating breast cancer is cytotoxic drug chemotherapy, which includes anthracyclines and albumin-binding paclitaxel [25, 26]. However, these conventional therapies have significant drawbacks, such as severe adverse effects and invasiveness, leading to a low 5-year survival rate and poor prognosis [27, 28]. Consequently, it is imperative to develop highly effective and low-toxicity treatments for breast cancer. Promising approaches include targeted cell cycle arrest, pathway/mRNA-directed therapies, immunomodulation, endocrine interventions, and integration of botanical agents as natural anticancer adjuvants. Collectively, these advances herald a paradigm shift toward durable disease control in breast cancer management.
In recent years, resveratrol has garnered substantial global scientific interest owing to its potent anti-tumor properties. Preclinical studies consistently demonstrate its efficacy against breast cancer through multifaceted mechanisms involving key molecular targets. These include modulation of cellular proliferation, suppression of epithelial-mesenchymal transition (EMT), enhancement of chemosensitization, inhibition of invasion and metastasis, induction of apoptosis, and epigenetic regulation [29, 30]. Emerging evidence positions resveratrol as a potent epigenetic modulator, exerting tumor-suppressive effects through promoter hypermethylation-mediated silencing of oncogenes and suppression of oncogenic Hedgehog and Wnt signaling pathways. This mechanistic profile underscores its potential as a strategic agent for breast cancer prevention and adjuvant therapy [6]. Numerous preclinical experiments have demonstrated that resveratrol inhibits the expression of histone transferase EZH2 by regulating the dephosphorylation of protein kinase ERK1/2, resulting in the inhibition of growth and proliferation of breast cancer cells [31]. Resveratrol also blocks the G0/G1 phase of breast cancer Michigan Cancer Foundation-7 (MCF-7) cells and reduces the expression of invasive and metastatic markers MMP2 and MMP9, thereby inhibiting the invasion and metastasis of breast cancer cells [32]. We observed the same inhibition of MMP2 and MMP9 proteins as described by the authors, further supporting the role of resveratrol in inhibiting breast cancer cell invasion and migration, although the potency varied across cell lines. Furthermore, resveratrol has been shown to inhibit the invasion and migration of human breast cancer MCF-7 cells through the PI3K/Akt and Wnt/β-catenin signaling pathways [33, 34]. Resveratrol has been found to reduce the expression of DNA polymerase delta 1 catalytic subunit(POLD1), inhibiting PCNA and Bcl-2 expression, while increasing the expression of the apoptotic index caspase-3; these effects activate the apoptotic pathway, promoting apoptosis in breast cancer cells [35]. Surprisingly, this finding can be confirmed by our experimental results that resveratrol can reduce breast cancer cell proliferation and induce apoptosis. Resveratrol exhibits a range of pharmacological activities and shows promising potential in the field of medicine and health care. However, in an earlier phase I clinical trial involving oral resveratrol (single doses of 0.5, 1, 2.5, or 5 g) in 10 healthy volunteers, resveratrol and its metabolites were excreted rapidly in urine, with 77% of urinary drug-derived species excreted within 4 h after the lowest dose, and resveratrol did not cause serious adverse events [36]. In another clinical study, patients were treated with doses ranging from 150 to 2,000 mg/day, showing improvements in insulin sensitivity, fasting glucose, or glycosylated hemoglobin (HbA1c) at doses of 150 - 500 mg/day [37]. After volunteers successfully completed 26 weeks of resveratrol (200 mg/day) intake, resveratrol supplementation improved memory in older adults, while improving glucose metabolism and increasing connectivity of hippocampal terminal fiber gray matter neurons [38]. In addition, a new study shows that although resveratrol has had very limited use in early clinical practice, a growing number of clinical trials support the benefits of resveratrol in the treatment of chronic diseases, which needs to be verified by testing new innovative formulations to enhance its pharmacokinetic profile [39]. In conclusion, there is no universal therapeutic concentration of resveratrol in clinical application, and its therapeutic concentration is highly dependent on the target disease, specific mechanism of action, administration schedule (dose, course of treatment), preparation form, and individual differences. However, the clinical translation of resveratrol remains constrained by pharmacokinetic limitations, including low oral bioavailability, suboptimal aqueous solubility, and chemical instability. Researchers face the challenging task of modifying its structure to develop resveratrol derivatives with enhanced biological activity and higher bioavailability.
PGK1, the first key enzyme in the glycolytic pathway, plays a crucial role in ATP production, breast cancer cell growth, and lactate production. Its multifaceted roles establish PGK1 as a promising molecular target for breast cancer therapeutic intervention [40, 41]. Notably, PGK1 acts not only as a metabolic enzyme but also as a protein kinase, facilitating tumor growth, migration, and invasion by phosphorylating essential substrates [42, 43]. Previous studies demonstrate that miR-16-1-3P directly targets the 3'-UTR of PGK1 mRNA, suppressing its translation. This post-transcriptional regulation attenuates glycolytic flux, resulting in decreased glucose uptake, lactate secretion, ATP generation, and extracellular acidification. Consequently, miR-16-1-3P effectively inhibits aerobic glycolysis and impairs tumor cell proliferation, invasion, and metastasis [44]. Additionally, acetylation at PGK1-323 K enhanced PGK1’s metabolic role by boosting its activity, glucose uptake, ATP production, and lactate output. This modification also promoted breast cancer cell proliferation, as evidenced by increased viability, S phase ratio, clonality, and PCNA levels [45]. Resveratrol also suppresses HK2-mediated glycolysis in non-small cell lung cancer (NSCLC) via inhibiting the AKT signaling pathway. This indicates that targeting HK2 or its upstream regulators represents a novel therapeutic strategy for NSCLC prevention and treatment [46]. In conclusion, targeting PGK1 expression presents a novel strategy to inhibit tumor growth, making it a potential biomarker for targeted tumor therapy.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by grants from the Natural Science Foundation of Heilongjiang Province (ZD2023H007), National Natural Science Foundation of China (No. 81972491), and the Qiqihar Science and Technology Plan Joint Guidance Project (No. LSFGG-2022033).
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent to participate in this study was provided by all patients.
Author Contributions
Yu Gao: writing original draft; conceptualization. Yao Yao Wang: investigation. Bao Di Wang: conceptualization. Qun Ying Hu: investigation. Ji Rui Jiang: investigation. Bo Feng: investigation. Xiu Li Gao: writing review and editing; funding acquisition. Li Kun Liu: data curation; funding acquisition. Wen Bin Zhu: conceptualization. Li Ling Yue: writing review and editing; funding acquisition.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Zhang X, Ge X, Jiang T, Yang R, Li S. Research progress on immunotherapy in triple-negative breast cancer (Review). Int J Oncol. 2022;61(2):95.
doi pubmed - Chhabra G, Singh CK, Amiri D, Akula N, Ahmad N. Recent advancements on immunomodulatory mechanisms of resveratrol in tumor microenvironment. Molecules. 2021;26(5):1343.
doi pubmed - Zaffaroni N, Beretta GL. Resveratrol and prostate cancer: the power of phytochemicals. Curr Med Chem. 2021;28(24):4845-4862.
doi pubmed - Pasquariello R, Verdile N, Brevini TAL, Gandolfi F, Boiti C, Zerani M, Maranesi M. The role of resveratrol in mammalian reproduction. Molecules. 2020;25(19):4554.
doi pubmed - Fu X, Li M, Tang C, Huang Z, Najafi M. Targeting of cancer cell death mechanisms by resveratrol: a review. Apoptosis. 2021;26(11-12):561-573.
doi pubmed - Kurzava Kendall L, Ma Y, Yang T, Lubecka K, Stefanska B. Epigenetic effects of resveratrol on oncogenic signaling in breast cancer. Nutrients. 2024;16(5):699.
doi pubmed - Cui J, Chai S, Liu R, Shen G. Targeting PGK1: a new frontier in breast cancer therapy under hypoxic conditions. Curr Issues Mol Biol. 2024;46(11):12214-12229.
doi pubmed - Liu H, Chen X, Wang P, Chen M, Deng C, Qian X, Bai J, et al. PRMT1-mediated PGK1 arginine methylation promotes colorectal cancer glycolysis and tumorigenesis. Cell Death Dis. 2024;15(2):170.
doi pubmed - Li X, Zheng Y, Lu Z. PGK1 is a new member of the protein kinome. Cell Cycle. 2016;15(14):1803-1804.
doi pubmed - Yang H, Geng YH, Wang P, Zhou YT, Yang H, Huo YF, Zhang HQ, et al. Extracellular ATP promotes breast cancer invasion and epithelial-mesenchymal transition via hypoxia-inducible factor 2alpha signaling. Cancer Sci. 2019;110(8):2456-2470.
doi pubmed - Ye T, Liang Y, Zhang D, Zhang X. MicroRNA-16-1-3p represses breast tumor growth and metastasis by inhibiting PGK1-mediated warburg effect. Front Cell Dev Biol. 2020;8:615154.
doi pubmed - Fu D, He C, Wei J, Zhang Z, Luo Y, Tan H, Ren C. PGK1 is a potential survival biomarker and invasion promoter by regulating the HIF-1alpha-mediated epithelial-mesenchymal transition process in breast cancer. Cell Physiol Biochem. 2018;51(5):2434-2444.
doi pubmed - http://gepia.cancer-pku.cn.
- http://www.proteinatlas.org.
- http://zinc.docking.org.
- Singh I, Seth A, Billesbolle CB, Braz J, Rodriguiz RM, Roy K, Bekele B, et al. Structure-based discovery of conformationally selective inhibitors of the serotonin transporter. Cell. 2023;186(10):2160-2175.e2117.
doi pubmed - Xu D, Aka JA, Wang R, Lin SX. 17beta-hydroxysteroid dehydrogenase type 5 is negatively correlated to apoptosis inhibitor GRP78 and tumor-secreted protein PGK1, and modulates breast cancer cell viability and proliferation. J Steroid Biochem Mol Biol. 2017;171:270-280.
doi pubmed - Wang L, Bo X, Yi X, Xiao X, Zheng Q, Ma L, Li B. Exosome-transferred LINC01559 promotes the progression of gastric cancer via PI3K/AKT signaling pathway. Cell Death Dis. 2020;11(9):723.
doi pubmed - Tang J, Qing MF, Li M, Gao Z. Dexamethasone inhibits BMP7-induced osteogenic differentiation in rat dental follicle cells via the PI3K/AKT/GSK-3beta/beta-catenin pathway. Int J Med Sci. 2020;17(17):2663-2672.
doi pubmed - Tang W, Wu Y, Qi X, Yu R, Lu Z, Chen A, Fan X, et al. PGK1-coupled HSP90 stabilizes GSK3beta expression to regulate the stemness of breast cancer stem cells. Cancer Biol Med. 2021;19(4):486-503.
doi pubmed - Chu Z, Huo N, Zhu X, Liu H, Cong R, Ma L, Kang X, et al. FOXO3A-induced LINC00926 suppresses breast tumor growth and metastasis through inhibition of PGK1-mediated Warburg effect. Mol Ther. 2021;29(9):2737-2753.
doi pubmed - Sun Y, Zhou QM, Lu YY, Zhang H, Chen QL, Zhao M, Su SB. RETRACTED: resveratrol inhibits the migration and metastasis of MDA-MB-231 human breast cancer by reversing TGF-beta1-induced epithelial-mesenchymal transition. Molecules. 2019;24(6):1131.
doi pubmed - Karim AM, Eun Kwon J, Ali T, Jang J, Ullah I, Lee YG, Park DW, et al. Triple-negative breast cancer: epidemiology, molecular mechanisms, and modern vaccine-based treatment strategies. Biochem Pharmacol. 2023;212:115545.
doi pubmed - Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, Zeng HM, et al. [Cancer incidence and mortality in China, 2022]. Zhonghua Zhong Liu Za Zhi. 2024;46(3):221-231.
doi pubmed - Yuan JQ, Wang SM, Guo L. S100A9 promotes glycolytic activity in HER2-positive breast cancer to induce immunosuppression in the tumour microenvironment. Heliyon. 2023;9(2):e13294.
doi pubmed - Li W, Xu M, Li Y, Huang Z, Zhou J, Zhao Q, Le K, et al. Comprehensive analysis of the association between tumor glycolysis and immune/inflammation function in breast cancer. J Transl Med. 2020;18(1):92.
doi pubmed - Narayan AK, Lee CI, Lehman CD. Screening for breast cancer. Med Clin North Am. 2020;104(6):1007-1021.
doi pubmed - Knisely J. Screening for breast cancer brain metastases. Lancet Oncol. 2022;23(5):e200.
doi pubmed - Hu C, Liu Y, Teng M, Jiao K, Zhen J, Wu M, Li Z. Resveratrol inhibits the proliferation of estrogen receptor-positive breast cancer cells by suppressing EZH2 through the modulation of ERK1/2 signaling. Cell Biol Toxicol. 2019;35(5):445-456.
doi pubmed - Jiang L, Yu H, Wang C, He F, Shi Z, Tu H, Ning N, et al. The anti-cancer effects of mitochondrial-targeted triphenylphosphonium-resveratrol conjugate on breast cancer cells. Pharmaceuticals (Basel). 2022;15(10):1271.
doi pubmed - Tang FY, Su YC, Chen NC, Hsieh HS, Chen KS. Resveratrol inhibits migration and invasion of human breast-cancer cells. Mol Nutr Food Res. 2008;52(6):683-691.
doi pubmed - Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH, Way TD, Chen WJ. 3,5,4'-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/beta-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol Appl Pharmacol. 2013;272(3):746-756.
doi pubmed - Suh J, Kim DH, Surh YJ. Resveratrol suppresses migration, invasion and stemness of human breast cancer cells by interfering with tumor-stromal cross-talk. Arch Biochem Biophys. 2018;643:62-71.
doi pubmed - Liang ZJ, Wan Y, Zhu DD, Wang MX, Jiang HM, Huang DL, Luo LF, et al. Resveratrol mediates the apoptosis of triple negative breast cancer cells by reducing POLD1 expression. Front Oncol. 2021;11:569295.
doi pubmed - Liu H, Wang X, Shen P, Ni Y, Han X. The basic functions of phosphoglycerate kinase 1 and its roles in cancer and other diseases. Eur J Pharmacol. 2022;920:174835.
doi pubmed - Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1246-1252.
doi pubmed - Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612-622.
doi pubmed - Witte AV, Kerti L, Margulies DS, Floel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34(23):7862-7870.
doi pubmed - Berretta M, Bignucolo A, Di Francia R, Comello F, Facchini G, Ceccarelli M, Iaffaioli RV, et al. Resveratrol in cancer patients: from bench to bedside. Int J Mol Sci. 2020;21(8):2945.
doi pubmed - He Y, Luo Y, Zhang D, Wang X, Zhang P, Li H, Ejaz S, et al. PGK1-mediated cancer progression and drug resistance. Am J Cancer Res. 2019;9(11):2280-2302.
pubmed - Gou R, Hu Y, Liu O, Dong H, Gao L, Wang S, Zheng M, et al. PGK1 is a key target for anti-glycolytic therapy of ovarian cancer: based on the comprehensive analysis of glycolysis-related genes. Front Oncol. 2021;11:682461.
doi pubmed - Li F, Ng WL, Luster TA, Hu H, Sviderskiy VO, Dowling CM, Hollinshead KER, et al. Epigenetic CRISPR screens identify Npm1 as a therapeutic vulnerability in non-small cell lung cancer. Cancer Res. 2020;80(17):3556-3567.
doi pubmed - Zhang J, Zhang J, Wei Y, Li Q, Wang Q. ACTL6A regulates follicle-stimulating hormone-driven glycolysis in ovarian cancer cells via PGK1. Cell Death Dis. 2019;10(11):811.
doi pubmed - Ye T, Liang Y, Zhang D, Zhang X. Corrigendum: MicroRNA-16-1-3p represses breast tumor growth and metastasis by inhibiting PGK1-mediated warburg effect. Front Cell Dev Biol. 2021;9:649787.
doi pubmed - Gao X, Pan T, Gao Y, Zhu W, Liu L, Duan W, Han C, et al. Acetylation of PGK1 at lysine 323 promotes glycolysis, cell proliferation, and metastasis in luminal A breast cancer cells. BMC Cancer. 2024;24(1):1054.
doi pubmed - Li W, Ma X, Li N, Liu H, Dong Q, Zhang J, Yang C, et al. Resveratrol inhibits Hexokinases II mediated glycolysis in non-small cell lung cancer via targeting Akt signaling pathway. Exp Cell Res. 2016;349(2):320-327.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.