| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 3, June 2025, pages 286-294
Time-to-Treatment Initiation and Its Effect on All-Cause Mortality: Insights From the Surveillance, Epidemiology, and End Results Database
Song Peng Anga, b, Eunseuk Leea, Jia Ee Chiab, Maya Iglesiasc, Mariela Di Vannaa, Shreya Shambhavia, Jose Iglesiasa, d, e, f
aDepartment of Medicine, Rutgers Health/Community Medical Center, Toms River, NJ, USA
bDivision of Cardiology, Sarver Heart Center, University of Arizona, Tucson, AZ, USA
dDepartment of Medicine, Texas Tech University Health Science Center, El Paso, TX, USA
cRutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA
eDepartment of Medicine, Hackensack Meridian School of Medicine, Nutley, NJ, USA
fCorresponding Author: Jose Iglesias, Department of Medicine, Hackensack Meridian School of Medicine, Nutley, NJ, USA
Manuscript submitted April 1, 2025, accepted May 29, 2025, published online June 14, 2025
Short title: TTI and Mortality in Cancer
doi: https://doi.org/10.14740/wjon2584
| Abstract | ▴Top |
Background: Delays in cancer treatment initiation can significantly impact survival outcomes, but the magnitude of this effect varies by cancer type, stage, and patient demographics. This study examined the association between time-to-treatment initiation (TTI) and all-cause mortality across multiple common cancers, evaluating differential impacts and sociodemographic disparities.
Methods: A retrospective cohort analysis was conducted using the Surveillance, Epidemiology, and End Results (SEER) database, including 991,771 adults diagnosed with breast, lung, prostate, or colorectal cancers between 2015 and 2020. TTI intervals were divided into four categories: 0 - 1, 2 - 5, 6 - 9, and ≥ 10 months. Cox proportional hazards models, adjusted for demographic, socioeconomic, cancer-specific, and treatment factors, assessed the impact of TTI on all-cause mortality, accounting for time-varying covariates.
Results: Overall, 63.9% of patients initiated treatment within 1 month. Unadjusted analyses revealed paradoxically lower mortality with longer TTI intervals (26.1% for 0 - 1 month vs. 11.4% for ≥ 10 months). After adjusting for time-varying effects, longer TTI significantly correlated with higher mortality risks (hazard ratio (HR): 1.02 for 2 - 5 months, 1.08 for 6 - 9 months, 1.23 for ≥ 10 months; P < 0.001 each), compared to treatment within 1 month. Older age (HR: 1.06), male gender (HR: 1.08), unmarried status (HR: 1.06), and non-Hispanic Black race (HR: 1.03) were independently associated with increased mortality. Lung cancer patients had significantly higher mortality than breast, prostate, and colorectal cancers (all P < 0.001). Treatment differences emerged, with reduced chemotherapy (40.2% to 10.0%) and surgical interventions (70.6% to 48.8%) at longer intervals.
Conclusion: Our analysis showed that increased TTI is independently associated with significantly higher all-cause mortality across major cancers, emphasizing the urgency of timely treatment initiation. Sociodemographic disparities in TTI and outcomes highlight systemic barriers disproportionately affecting vulnerable populations, necessitating targeted interventions to improve equitable cancer care and survival outcomes.
Keywords: Mortality; Time-to-treatment initiation; Breast cancer; Lung cancer; Prostate cancer; Colorectal cancer
| Introduction | ▴Top |
Time-to-treatment initiation (TTI) represents a critical window between cancer diagnosis and the start of definitive treatment that can significantly impact patient outcomes [1]. Understanding the factors influencing TTI and its association with mortality is crucial for optimizing cancer care and addressing health disparities. Lung, breast, colorectal, and prostate cancers are among the most common malignancies worldwide, accounting for a substantial proportion of cancer-related deaths [2, 3]. In the United States, these four cancer types collectively represent approximately 45% of all new cancer cases and 40% of cancer-related deaths [4]. Despite advances in screening and treatment, disparities in cancer outcomes persist across various demographic groups.
The relationship between TTI and mortality is complex and could be potentially modified by numerous factors, including cancer type, stage at diagnosis, and patient characteristics [5]. Sociodemographic factors such as race, age, gender, socioeconomic status, and geographic location have been documented to influence both TTI and mortality outcomes [1, 6]. These disparities often mirror broader healthcare inequities and may contribute to observed differences in cancer survival rates among diverse populations. Despite growing recognition of TTI’s importance, comprehensive analyses examining the relationship between TTI, sociodemographic factors, and mortality across multiple common cancers remain limited. Most studies have focused on single cancer types or specific demographic factors, leaving gaps in our understanding of how TTI impacts mortality across different patient populations and cancer types [7].
The primary aim of this study was to investigate the association between TTI and mortality in the four most common cancer types: lung cancer, breast cancer, colorectal cancer, and prostate cancer. We hypothesized that delay in TTI is associated with increased mortality while accounting for sociodemographic factors.
| Materials and Methods | ▴Top |
Data source and patient selection
We retrospectively utilized the Surveillance, Epidemiology, and End Results (SEER) database from the year 2015 to 2020. Adults aged 20 and older diagnosed with lung cancer, breast cancer, colorectal cancer, or prostate cancer were identified. Specifically, we created a session to evaluate individual cancer cases utilizing data from Incidence - SEER Research Data, 17 Registries, Nov 2022. Patients with unknown TTI or mortality status were excluded from the analysis. We excluded pediatric patients and cases diagnosed outside the studied time frame.
Ethical issues
This research study was approved by local Institutional Review Board (IRB), Rutgers Health IRB #24-001. The study was performed according to the regulations established by the Clinical Research and Ethics Committee and to the Helsinki Declaration of the World Medical Association.
Variables selection
The primary exposure variable was TTI, measured in months from diagnosis to first treatment. The primary outcome variable was all-cause mortality, defined as death attributed to any causes within the follow-up period. Variables extracted included sex, age, ethnicity, marital status, median household income, geographic location, cancer type, stage of cancer, grade of cancer, and modality of treatment, i.e., surgery, chemotherapy, or radiotherapy.
Data analysis
Categorical variables were reported as numbers and percentages and analyzed using the Pearsons Chi-square test. A statistically significant value was defined as P less than 0.05. Those variables found to be significant on univariate analysis were entered into a multivariable Cox proportional hazards model, specifically, stage of cancer, grade, radiation treatment and chemotherapy, and TTI as time-dependent covariates. Statistics were performed with STATA (version 17.0; StataCorp LLC, College Station, TX).
| Results | ▴Top |
In this study, we analyzed a cohort of 991,771 participants across four TTI intervals, with 633,943 (63.9%) initiating therapy within 0 - 1 months, 330,596 (33.3%) within 2 - 5 months, 21,120 (2.1%) within 6 - 9 months, and 6,112 (0.6%) at ≥ 10 months post-diagnosis (Table 1). The age distribution remained relatively consistent, with approximately 45% of participants under 65 years and 55% aged 65 or older across all intervals. However, significant sex differences emerged, transitioning from female predominance (63.2%) in the 0 - 1 month cohort to male predominance (79.4%) in the ≥ 10 months group. Racial and ethnic composition also shifted longitudinally, with decreasing representation of non-Hispanic White patients (from 69.5% to 58.0%) and increasing proportions of non-Hispanic Black (from 10.5% to 19.3%) and Hispanic (from 10.4% to 13.5%) populations with the increase in TTI intervals.
 Click to view | Table 1. Baseline Characteristics of Participants by Time-to-Treatment Initiation (TTI) |
Socioeconomic analysis revealed persistent disparities, with 48.0-49.7% of patients across all TTI groups reporting household incomes ≥ $75,000 annually. Metropolitan residence prevalence increased from 86.8% to 91.2% with longer TTI. In terms of cancer-specific factors, localized stage malignancies increased from 48.8% (0 - 1 months) to 69.6% (≥ 10 months), while distant metastases decreased from 22.2% to 7.6%. Treatment patterns showed reduced chemotherapy utilization (from 40.2% to 10.0%) and surgical intervention (from 70.6% to 48.8%) with prolonged TTI, though radiation therapy remained consistently high at approximately 97% across TTI intervals. Cancer type distribution showed marked temporal variation; breast (40.8%) and lung (22.5%) cancers predominated early TTI intervals (0 - 1 month), while prostate cancer prevalence increased substantially from 12.3% to 69.1% in later TTI intervals.
All-cause mortality
Prior to adjusting for sociodemographic factors, cancer-specific factors and time-varying covariates, the rate of all-cause mortality stratified by TTI are as follows: 26.1% for 0 - 1 month, 15.9% for 2 - 5 months, 12% for 6 - 9 months, and 11.4% for ≥ 10 months (Table 2) (Fig. 1, Supplementary Materials 1-3, wjon.elmerpub.com). The Cox proportional hazards model revealed that TTI has a significant association with mortality. Compared to patients with TTI of 0 - 1 months (reference group), those with longer TTI had progressively lower hazard ratios (HRs) for mortality: 2 - 5 months (HR: 0.90, 95% confidence interval (CI): 0.88 - 0.92), 6 - 9 months (HR: 0.78, 95% CI: 0.72 - 0.85), and ≥ 10 months (HR: 0.52, 95% CI: 0.45 - 0.61), all P < 0.001. However, when accounting for time-varying effects, longer TTI was associated with increased mortality risk: 2 - 5 months (HR: 1.02, 95% CI: 1.01 - 1.03), 6-9 months (HR: 1.08, 95% CI: 1.04 - 1.12), and ≥ 10 months (HR: 1.23, 95% CI: 1.16 - 1.31, P < 0.001) (Table 3).
 Click to view | Table 2. Overall Mortality and Mortality by Type of Cancer |
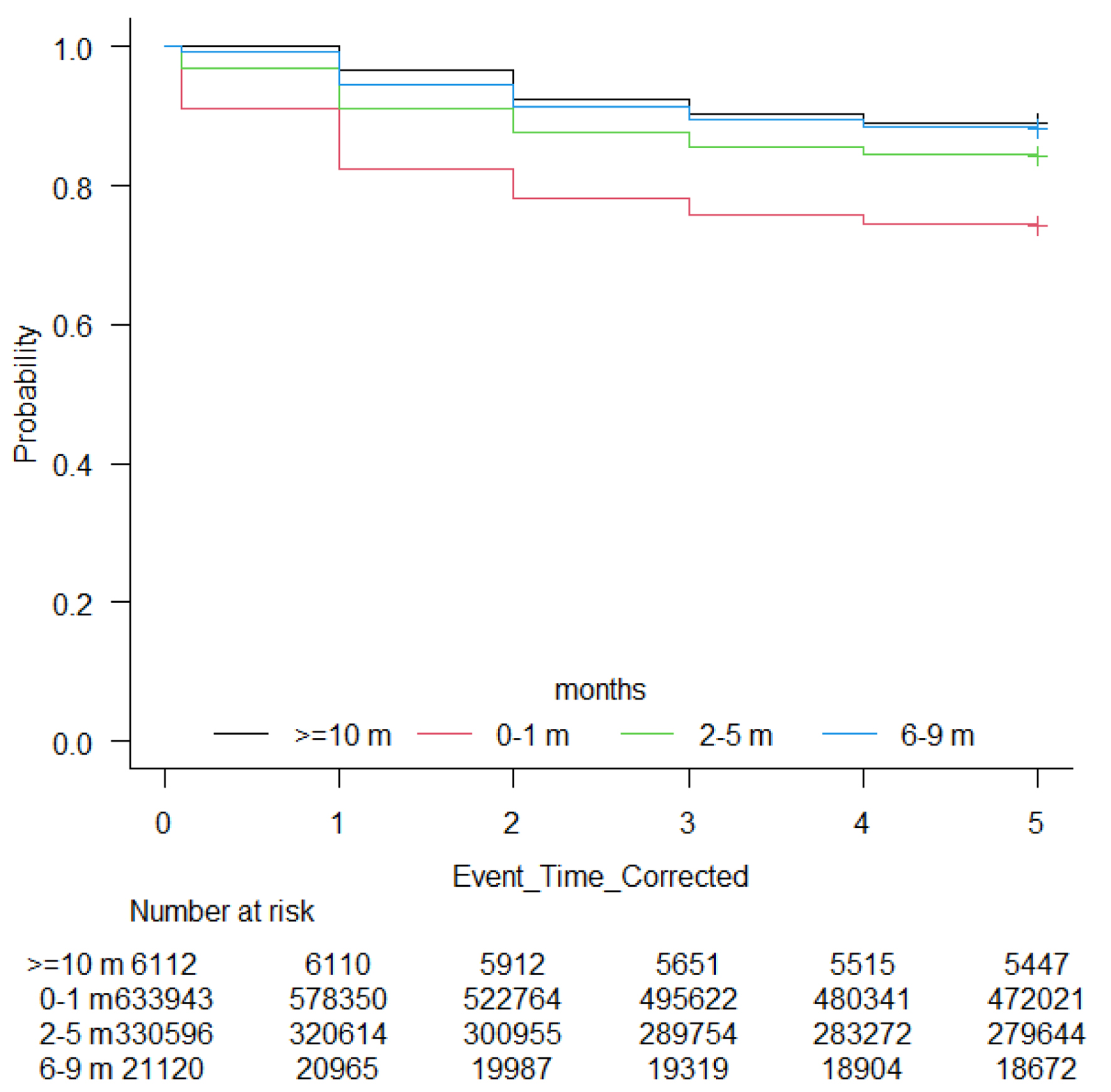 Click for large image | Figure 1. Kaplan-Meier curve showing overall survival by time-to-treatment initiation (TTI). |
 Click to view | Table 3. Cox Proportional Hazard Model Accounting for Time-Varying Covariates |
Other significant factors associated with increased mortality risk included age ≥ 65 (HR: 1.06, 95% CI: 1.05 - 1.08), male sex (HR: 1.08, 95% CI: 1.07 - 1.09), and being separated (HR: 1.04, 95% CI: 1.03 - 1.06) or single/unmarried/widowed (HR: 1.06, 95% CI: 1.05 - 1.08) compared to married individuals. Non-Hispanic Black patients had a slightly higher risk (HR: 1.03, 95% CI: 1.02 - 1.05, P < 0.001) compared to non-Hispanic Whites, while non-Hispanic Asian or Pacific Islanders had a lower risk (HR: 0.94, 95% CI: 0.92 - 0.96, P < 0.001).
Regarding cancer types, breast (HR: 0.81, 95% CI: 0.80 - 0.83), prostate (HR: 0.67, 95% CI: 0.66 - 0.69), and colon (HR: 0.95, 95% CI: 0.94 - 0.97) cancers had lower mortality risks compared to lung cancer (all P < 0.001). Surgery was associated with lower mortality risk (HR: 0.84, 95% CI: 0.83 - 0.85, P < 0.001). Higher grade tumors were associated with lower initial mortality risk but higher time-varying risk. Chemotherapy was associated with lower initial risk (HR: 0.58, 95% CI: 0.57 - 0.59, P < 0.001) but higher time-varying risk (HR: 1.03, 95% CI: 1.02 - 1.04, P < 0.001).
| Discussion | ▴Top |
This large-scale analysis of TTI and mortality across four common cancer types reveals a complex relationship between treatment delays and patient outcomes. Our findings demonstrate that after accounting for time-varying effects, longer TTI was significantly associated with increased mortality risk, with HRs progressively rising from 1.02 for 2 - 5 months to 1.23 for delays of ≥ 10 months when compared to treatment within 1 month of diagnosis. This temporal relationship highlights the critical importance of timely initiation of cancer therapy, particularly for certain cancer types and demographic groups.
The paradoxical observation of lower unadjusted mortality rates with longer TTI (26.1% for 0 - 1 month vs. 11.4% for ≥ 10 months) likely reflects treatment selection bias, whereby patients with more aggressive disease or worse performance status receive expedited care. This phenomenon has been documented in previous studies and emphasizes the importance of multivariate analyses that account for time-varying covariates. After appropriate adjustment, our results align with growing evidence that treatment delays are associated with worse outcomes. Previous research has demonstrated that each 4-week delay in cancer treatment is associated with a 6-13% increase in mortality risk, with even greater impacts for specific cancer types and treatment modalities [8].
Our findings reveal significant sociodemographic disparities in both TTI and subsequent mortality. We observed a shift from female predominance in the earliest treatment group to male predominance in the most delayed group, suggesting potential sex-based disparities in access to timely care. Similarly concerning was the decreasing representation of non-Hispanic White patients and increasing proportions of non-Hispanic Black and Hispanic populations with longer TTI intervals. These patterns reflect broader healthcare inequities that have been documented in numerous studies, including recent work identifying insurance status, language barriers, and socioeconomic status as predictors of delayed care [9-11]. For instance, Smith et al studied the relationship between treatment delay time and 5-year survival rates using the California Cancer Registry [12]. They reported that women with public or no insurance and women (17.8% compared to 9.5% with private insurance) with low socioeconomic status (17.5% compared to 7.7% with high socioeconomic status) were associated with treatment delay time of more than 6 weeks more frequently [12].
Importantly, the 5-year survival rate for women with surgical treatment delays beyond 6 weeks was 80% compared to 90% for those treated within 2 weeks. Eaglehouse et al studied the time-to-breast cancer surgery in the Military Health System. They found that while the median time to surgery was similar (21 days for White women versus 22 days for Black women), Black women experienced significantly greater delays at the 75th (3.6 days longer) and 90th (8.9 days longer) percentiles compared to White women [13].
The relationship between TTI and mortality varied substantially by cancer type, reflecting differences in biology, treatment approaches, and staging patterns. While direct comparisons between cancer types were not the primary focus of our analysis, we noted that breast, prostate, and colorectal cancers had lower mortality risks compared to lung cancer after controlling for TTI and other factors. This is in contrast with prior research demonstrating varying sensitivities to treatment delays across cancer types, with colon cancer having the most pronounced mortality association with TTI, while prostate cancer was least associated [14]. Interestingly, our analysis revealed that localized stage malignancies increased from 48.8% in the earliest treatment group to 69.6% in the most delayed group, while distant metastases decreased from 22.2% to 7.6%. This pattern may partly reflects appropriate clinical prioritization, with advanced disease receiving more urgent care. However, it also raises concerns about the potential impact of delays on stage migration and disease progression during the waiting period, a phenomenon that our study was not designed to directly quantify but our time-varying analysis provided indirect findings that such progression contributes to worse outcomes.
To clarify the impact of stage dynamics, we specified stage of cancer as time-varying covariates in our extended Cox model. At baseline, distant metastases conferred the greatest early hazard, whereas localized and regional disease showed lower initial risk. Over follow-up, however, these “favorable” stages experienced rising hazards, suggesting biologic progression while patients awaited therapy. Conversely, the apparent survival advantage of longer TTI in unadjusted analyses largely disappeared once stage evolution was accounted for, reinforcing that delays may permit up-staging rather than merely reflecting case-mix. Taken together, these findings argue for diagnostic pathways that complete essential staging rapidly so that definitive treatment can begin before tumor burden increases.
Treatment patterns varied significantly with TTI, with reduced utilization of chemotherapy and surgery in patients with longer delays. This may reflect both appropriate clinical decision-making and concerning barriers to comprehensive cancer care. The consistently high utilization of radiation therapy across all TTI intervals (approximately 97%) is noteworthy and merits further study to understand the factors supporting this consistency versus the variability observed in other treatment modalities.
The negative impact of treatment delays is particularly pronounced in early-stage cancers where curative-intent therapy offers the greatest potential benefit. For example, in stage I breast cancer, treatment delays ≥ 6 months were associated with a 23% increased mortality risk compared to immediate treatment [15]. This increased sensitivity in early-stage disease aligns with findings from a comprehensive systematic review showing shorter TTI significantly improves survival outcomes across multiple cancer types [14]. The relationship between diagnostic delays and outcomes is particularly evident in symptomatic presentations. A systematic review of 209 studies encompassing 177 articles found consistent associations between shorter time-to-diagnosis and improved survival for breast, colorectal, head and neck, testicular cancers, and melanoma [16]. This review highlighted that each month of delay in surgical treatment correlated with 6-8% increased mortality risk, while delays in radiotherapy and systemic therapies showed even greater impacts (9-13% increased risk per month).
Limitations
Several limitations warrant consideration when interpreting our findings. First, the retrospective nature of this study using the SEER database introduces potential confounding biases. While we adjusted for numerous covariates, unmeasured, residual confounding remains possible, particularly regarding factors influencing treatment decisions that are not captured in administrative databases. Second, our definition of TTI as time from diagnosis to first treatment does not account for potential delays in diagnosis following symptom onset or screening abnormalities. The total cancer care continuum includes multiple time points, and our study addresses only one segment of potential delays. Additionally, data including patients’ comorbidities, the type and size of the hospital as well as distance to the treatment center were not available in this study. Future research should evaluate the entire care continuum to identify additional opportunities for intervention. Third, while we examined all-cause mortality, we were unable to assess cancer-specific mortality or quality of life outcomes. Treatment delays may impact these endpoints differently, and future prospective studies incorporating these measures would provide a more comprehensive understanding of the consequences of delayed care. Fourth, our analysis focused on four common cancer types and may not generalize to all malignancies. Cancer-specific biological factors may influence the impact of treatment delays, and further research examining additional cancer types is warranted. Fifth, we did not study laboratory or imaging findings which may carry prognostic value, and this represents a potential avenue for future research. Finally, our study population was derived from the United States and may not be generalized to other regions of the world.
Conclusion
In conclusion, our study of TTI across four common cancer types reveals a significant association between treatment delays and increased mortality risk, even after adjusting for sociodemographic factors, cancer stage, and treatment modalities. Our findings highlight persistent disparities in TTI and mortality among different demographic groups, emphasizing the need to address systemic barriers to timely care. The impact of delays appears particularly pronounced in early-stage cancers, highlighting the importance of prompt intervention when curative treatment is most effective. While this research provides valuable insights into the relationship between TTI and cancer outcomes, it also identifies areas requiring further investigation, including the entire continuum of cancer care delays, cancer-specific and patient-reported outcomes. Ultimately, prioritizing equitable access to timely cancer care is crucial for improving survival outcomes and reducing healthcare disparities among these vulnerable populations.
| Supplementary Material | ▴Top |
Suppl 1. Kaplan-Meier Curve Showing Overall Survival of Lung Cancer by Time-to-Treatment Initiation (TTI).
Suppl 2. Kaplan-Meier Curve Showing Overall Survival of Breast Cancer by Time-to-Treatment Initiation (TTI).
Suppl 3. Kaplan-Meier Curve Showing Overall Survival of Prostate Cancer by Time-to-Treatment Initiation (TTI).
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
SPA: conceptualization, methodology, investigation, writing - original draft preparation, writing - reviewing and editing. EL: visualization, writing - original draft preparation, and writing - reviewing and editing. JEC: interpretation, visualization, writing - original draft preparation, and writing - reviewing and editing. MI: data acquisition and writing - original draft preparation. MDV: data acquisition, validation, and writing - original draft preparation. SS: data acquisition, validation, and writing - original draft preparation. JI: methodology, analysis, interpretation, writing - reviewing and editing, and supervision. All authors provided final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Data Availability
The data supporting this study are obtained from the SEERS database and are available upon application. Restrictions applied as these were used under license for this study.
| References | ▴Top |
- Di Vanna M, Shambhavi S, Khikmatov M, Ang SP, Iglesias J. Time to treatment initiation of lung, breast, colorectal, and prostate cancers and contributing factors from 2015 to 2020 utilizing surveillance, epidemiology, and end results program database. World J Oncol. 2025;16(2):152-160.
doi pubmed - Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Chia JE, Ang SP, Usman MH, Krittanawong C, Mukherjee D. Trends, characteristics and outcomes in breast cancer survivors with STEMI. Angiology. 2025.
doi pubmed - National Cancer I. SEER Cancer Statistics Factsheets: Common Cancer Sites. 2024.
- Cushman TR, Jones B, Akhavan D, Rusthoven CG, Verma V, Salgia R, Sedrak M, et al. The effects of time to treatment initiation for patients with non-small-cell lung cancer in the United States. Clin Lung Cancer. 2021;22(1):e84-e97.
doi pubmed - Bhatia RK, Rayne S, Rate W, Bakwenabatsile L, Monare B, Anakwenze C, Dhillon P, et al. Patient factors associated with delays in obtaining cancer care in Botswana. J Glob Oncol. 2018;4:1-13.
doi pubmed - Khanna S, Kim KN, Qureshi MM, Agarwal A, Parikh D, Ko NY, Rand AE, et al. Impact of patient demographics, tumor characteristics, and treatment type on treatment delay throughout breast cancer care at a diverse academic medical center. Int J Womens Health. 2017;9:887-896.
doi pubmed - Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E, O'Sullivan DE, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087.
doi pubmed - Sheni R, Qin J, Viswanathan S, Castellucci E, Kalnicki S, Mehta V. Predictive factors for cancer treatment delay in a racially diverse and socioeconomically disadvantaged urban population. JCO Oncol Pract. 2023;19(6):e904-e915.
doi pubmed - Stuart CM, Mott NM, Bronsert MR, Randhawa SK, David EA, Mitchell JD, Meguid RA. The association between sociodemographic factors and delays to minimally invasive surgery for stage IA-IIIA non-small cell lung cancer. Ann Thorac Surg. 2025;119(5):1082-1091.
doi pubmed - Berrian JL, Liu Y, Lian M, Schmaltz CL, Colditz GA. Relationship between insurance status and outcomes for patients with breast cancer in Missouri. Cancer. 2021;127(6):931-937.
doi pubmed - Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148(6):516-523.
doi pubmed - Eaglehouse YL, Georg MW, Shriver CD, Zhu K. Racial Differences in Time to breast cancer surgery and overall survival in the US military health system. JAMA Surg. 2019;154(3):e185113.
doi pubmed - Cone EB, Marchese M, Paciotti M, Nguyen DD, Nabi J, Cole AP, Molina G, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072.
doi pubmed - Min Y, Liu Z, Huang R, Li R, Jin J, Wei Z, He L, et al. Survival outcomes following treatment delays among patients with early-stage female cancers: a nationwide study. J Transl Med. 2022;20(1):560.
doi pubmed - Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, Hamilton W, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.