| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 3, June 2025, pages 276-285
Adjuvant Therapy Benefits for Patients With Human Epidermal Growth Factor Receptor 2-Positive T1aN0M0 Breast Cancer: A Systematic Review and Meta-Analysis
Ezzeldin M. Ibrahima, d, Ahmed A. Refaea, Ali M. Bayera, Nouf Abdullahb, Meteb E. Al-Foheidic
aOncology Department, King’s College Hospital London, Jeddah 23412, Saudi Arabia
bMedical Imaging Department and Breast Unit, King’s College Hospital London, Jeddah 23412, Saudi Arabia
cPrincess Noorah Oncology Center, King Abdulaziz Medical City, PO Box 9515, Jeddah 21423, Saudi Arabia
dCorresponding Author: Ezzeldin M. Ibrahim, Oncology Department, King’s College Hospital London, Jeddah, Saudi Arabia
Manuscript submitted March 15, 2025, accepted May 2, 2025, published online June 9, 2025
Short title: Adjuvant Therapy for HER2-Positive T1a Breast Cancer
doi: https://doi.org/10.14740/wjon2578
| Abstract | ▴Top |
Background: While the prognosis for patients with human epidermal growth factor receptor 2 (HER2)-positive pT1a-bN0M0 breast cancer is generally favorable, the optimal approach to personalize adjuvant treatment for T1a tumors remains unclear, which prompted an impetus to conduct a systematic review and meta-analysis for the latter group.
Methods: We examined the literature for studies that provided relevant data about HER2-positive T1a patients. Patient and disease characteristics, therapy details, and survival outcomes were extracted.
Results: Thirteen studies with 2,089 patients were eligible; four were prospective and nine were retrospective. In the studies where patients did not receive chemotherapy or anti-HER2 therapy, the prognosis was generally favorable, with disease-free survival (DFS) and overall survival of approximately 92% to 99%. Studies comparing treated versus untreated patients showed a survival benefit that varied between 2% and 15%, favoring adjuvant therapy without reaching statistical significance. In the only included randomized trial where all patients received adjuvant paclitaxel and trastuzumab, 10% demonstrated 5-year invasive DFS events. A meta-analysis of four studies showed a nonsignificant survival advantage trend among treated patients. There was inconsistency about the prognostic role of the co-existing hormone receptor status.
Conclusion: Patients with HER2-positive T1aN0 have a favorable prognosis; the benefit of adjuvant chemotherapy plus anti-HER2 varied and showed no convincing statistically significant benefit. The decision to offer adjuvant therapy should balance the expected benefits and risks. Prospective trials that include this population should be able to identify who should receive adjuvant therapy and determine the magnitude of benefit.
Keywords: Breast cancer; HER2-positive; Stage T1aN0; Adjuvant therapy; Survival; Systematic review; Meta-analysis
| Introduction | ▴Top |
Worldwide, the diagnosis of small breast carcinoma, specifically pT1a-bN0M0, has significantly increased with the advent of mammographic screening and prevention programs [1, 2]. For breast cancer screening, worldwide quality criteria suggest aiming for a prevalence of at least 20% T1ab in all detected tumors [3]. While the prognosis for patients with pT1a-bN0M0 breast cancer is generally favorable, the optimal approach to personalize adjuvant treatment remains unclear. While adjuvant therapy can benefit some patients, its absolute efficacy diminishes with smaller primary tumor sizes. Notably, approximately 20-30% of detected cancers are 1 cm or smaller, posing a challenge in deciding the necessity of adjuvant therapy [4].
Human epidermal growth factor receptor 2 (HER2)-positive breast cancers are associated with poorer disease-free survival (DFS) and overall survival (OS) rates [5]. The 5-year DFS in patients with HER2-positive small breast cancer treated with local therapy only ranged between 77% and 95% [6]. The HER2-positive phenotype is linked to poorly differentiated tumors, high proliferative rates, and the absence of estrogen and progesterone receptors.
Phase III clinical trials have shown significant improvements in survival with the addition of trastuzumab to adjuvant chemotherapy for early-stage, HER2-positive breast cancer [7-9]. However, these trials primarily included node-positive cases and excluded patients with 1 cm or smaller tumors; therefore, there is no high level of evidence that could guide decision-making concerning systemic adjuvant therapy for node-negative small tumors. Current National Comprehensive Cancer Network (NCCN) guidelines suggest considering adjuvant chemotherapy with trastuzumab with or without endocrine therapy (ET) based on the endocrine receptor (ER) status for pT1aN0M0 and recommend such treatment for pT1bN0M0 [10]. The European Society of Medical Oncology (ESMO) also made a similar recommendation with an emphasis on the use of less toxic protocols [11].
A recently reported systematic review and meta-analysis concluded that patients with HER2-positive T1abN0M0 achieved significantly improved DFS and OS treated with chemotherapy and trastuzumab compared to patients without chemotherapy and trastuzumab [12]. However, a conclusion about the benefit of such therapy for a subgroup of patients with T1aN0M0 tumors could not be ascertained.
This systematic review and meta-analysis address some critical questions. What are the survival outcomes of women with HER2-positive T1aN0M0? What is the benefit of adjuvant therapy for these patients? Can adjuvant therapy (trastuzumab or other anti-HER2 therapy with or without chemotherapy) be omitted in a subset of patients with HER2-positive cancers, and could associated ER status guide this decision?
| Materials and Methods | ▴Top |
Search strategy
Between January 2000 and October 2024, we identified studies of interest following an electronic literature search of the following databases: MEDLINE, EMBASE, and the Cochrane Library. Relevant abstracts from the American Society of Clinical Oncology and the ESMO conference proceedings were also explored.
Terms or keywords from the Medical Subject Headings were utilized: “breast”, “cancer OR neoplasm OR tumor OR carcinoma OR malignant”, “stage 1 OR stage I OR T1N0 OR T1abN0 OR T1aN0”, “trastuzumab AND/OR chemotherapy”, “clinical trial (mh) OR prospective study (mh) OR randomized controlled trial (mh) OR retrospective study OR cohort study OR comparative study OR follow-up study”. The terms were subsequently combined with the keywords “survival OR outcome OR death”.
Inclusion and exclusion criteria
We included all studies that satisfied the following criteria: 1) reported in the English language between January 2000 and October 2024; 2) included patients with HER2-positive T1aN0 tumors; 3) investigated and reported the outcomes of patients receiving or no-recipient of adjuvant systemic therapy other than ET, or having data allowing such outcomes to be derived; 4) any study design; and 5) published as original articles or abstracts. When more than one article reported duplicate data, we included the most recent and relevant; however, we included studies that used the same dataset but examined additional relevant outcomes. Studies were excluded if neoadjuvant anti-HER2 therapies were used; moreover, studies that reported combined results of T1a and T1b without distinction based on the tumor size were also excluded.
Data extraction
The authors independently inspected each identified and applied the inclusion and exclusion criteria, and the discussion resolved any discordance. The extracted data included the reported authors’ information, year of publication, study design, patient and disease characteristics, adjuvant therapy details, and patient outcomes.
Statistical analyses
For the meta-analysis, the primary endpoint was the pooled effect size of the hazard ratio (HR) for the survival difference between those who received adjuvant therapy and those who did not. If enough data were available, we calculated the unreported outcome and its 95% confidence interval (CI) by applying the methodology of Tierney et al [13], according to the method of Parmar et al [14].
Considering the assumed designs of the identified studies, the DerSimonian and Laird random-effects model was used [15]. Heterogeneity was determined by inspecting the graphical presentations and calculating a χ2 heterogeneity test and the I2 inconsistency statistics [16]. Statistically significant heterogeneity was defined as a χ2 P value less than 0.1 or an I2 statistic > 50%. If indicated, we planned a meta-regression analysis to identify clinical variables that could explain demonstrated heterogeneity. To perform weighted least-square linear regression, the dependent variable was the InHR weighted for the inverse of the variance. All statistical tests were two-sided. We used the Review Manager for Windows software version 5.4 (The Cochrane Collaboration, Oxford, UK) to compute pertinent data.
Review conduct and reporting data
The Cochrane Handbook’s recommendations were adopted in this systematic review, and the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement was followed in the report [17].
Ethical considerations
According to our institutional policy, IRB approval is not required for meta-analysis projects. A statement of ethics does not apply to this research because we reviewed and analyzed data from published research.
| Results | ▴Top |
In Figure 1, the flowchart of the selection process of study selection is shown. After excluding duplicate references, non-relevant literature, and those not satisfying the inclusion criteria, 13 studies were included [18-30]. Table 1 demonstrates the patient and disease characteristics of the included studies. The total number of patients was 2,089; however, the study by Joensuu et al reported 69 patients having T1a and T1b combined [18], and Gumucci et al reported 96 patients having T1a-c tumors together [21]. The patients’ median age ranged between 51.5 and 96 years when reported. The studies included a variable number of patients, and Kubo et al reported the study from Japan that included the largest number of patients (1,113 patients, all with T1 tumors) [29].
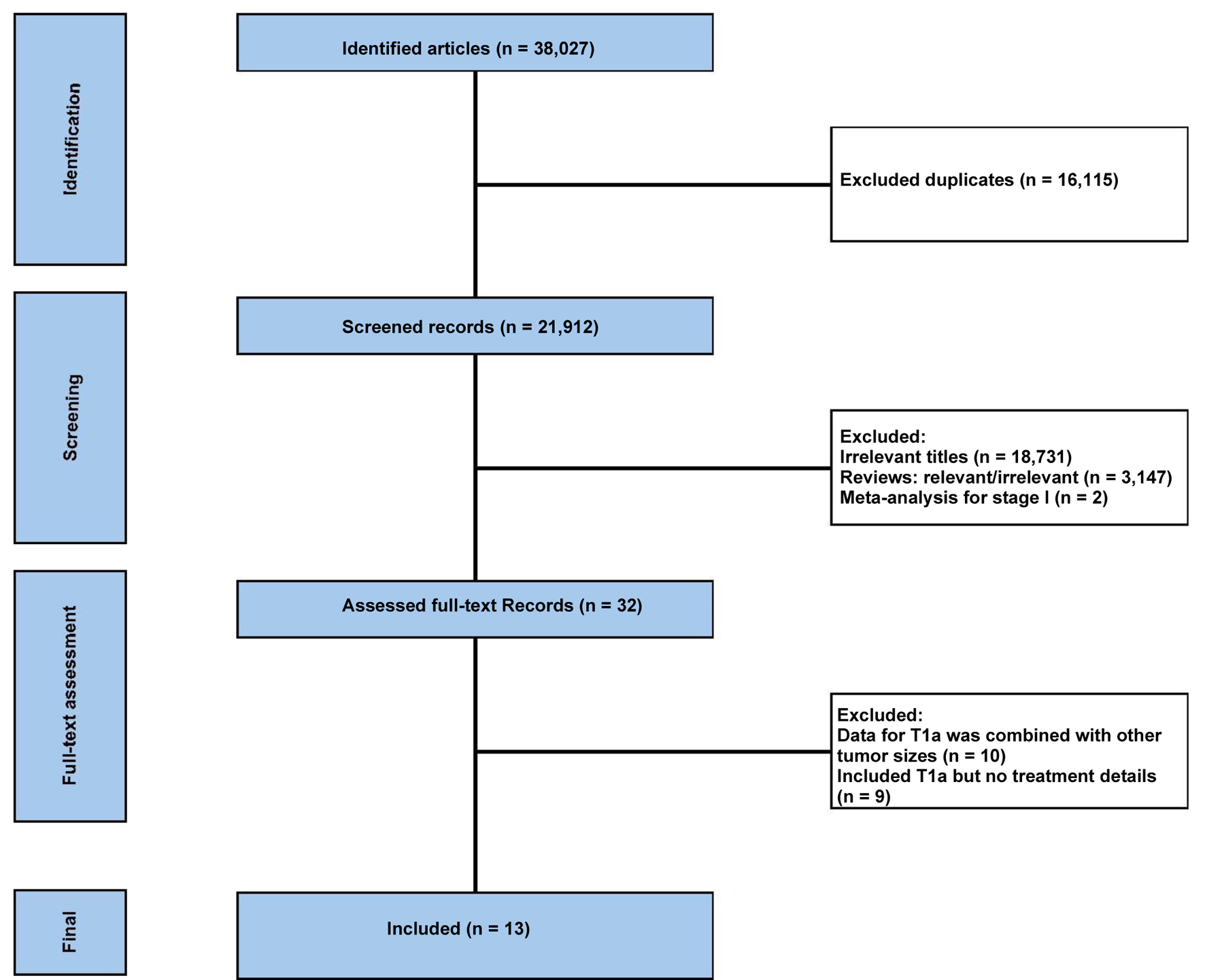 Click for large image | Figure 1. The flowchart of the study selection process. |
 Click to view | Table 1. Patient and Disease Characteristics of Patients With HER2-Positive T1aN0M0 Breast Cancer |
A brief summary of the study characteristics
The study by Joensuu et al [18] was based on data retrospectively reviewed by the Finnish Cancer Registry between 1991 and 1992. The study analyzed 852 women with T1N0M0 breast cancer; only 69 (12%) were HER2-positive, and none received adjuvant systemic therapy.
At the European Institute of Oncology, Curigliano et al [19] reviewed the data of 150 patients with pT1abNOM0 HER2-positive tumors who received no adjuvant trastuzumab.
From the Radiation-Oncology Center of Florence University (Florence, Italy) clinical database, Livi et al [20] reviewed data on 705 patients with T1abN0MO breast cancer, including 28 patients with HER2-positive T1a disease.
Gamucci et al [21] retrospectively reviewed 900 patients (T1a-c, node-negative) from four Italian centers. The authors reported on 97 patients with HER2-positive T1a-c, but the exact number of T1a was not reported. We elected to include this study because the report included the survival outcomes of HER2-positive T1a patients and their therapy details.
From the Kaiser Permanente Clinical Care Program of Northern California, USA, Fehrenbacher et al [22] reported on 56 and 118 patients with pT1abN0M0 and T1bN0M0 HER2-positive tumors, respectively. No adjuvant systemic therapy was offered to 100 (86%) and 71 (60%) patients with T1a and T1b, respectively.
Vaz-Luis et al reported a prospective cohort study within the NCCN database that included 4,113 women with T1a-bN0M0 breast cancer treated between 2000 and 2009 [23]. The dataset included 216 (5%) and 304 (7.4%) patients with HER2-positive T1a and T1b tumors, respectively.
van Ramshorst et al [24] identified 2,327 patients diagnosed with T1N0M0 HER2-positive invasive breast cancer from the Netherlands Cancer Registry, which included 385 (17%) patients with T1aN0 tumors. Forty-five percent of patients received chemotherapy with or without trastuzumab; 92% received both.
A German group reported a prospective noninterventional study on 4,027 patients with HER2-positive breast cancer disease, and the dataset included 97 (2.4%) patients with T1aN0 tumors [25].
de Nonneville et al [26] reported 356 cases of pT1abN0M0 HER2-positive breast cancers identified from 17 French cancer centers that included 138 patients with T1a tumors, and one-third received chemotherapy with or without trastuzumab.
Musolino et al [27] reported on 874 patients with pT1aN0M0 identified from the Italian Emilia-Romagna region. This dataset included 121 (14%) patients with HER2-positive T1aN0 disease.
He et al [28] reviewed the medical records of patients receiving care at MD Anderson Cancer Center in the USA between 1998 and 2009. Patients were categorized into three groups. Group A included patients with no adjuvant therapy, while group B and group C included patients who received adjuvant chemotherapy only and chemotherapy plus trastuzumab, respectively.
Kubo et al [29] published on 2,736 patients with HER2-positive, node-negative pT1a-c breast cancer identified from the Japan National Clinical Database. Eighteen percent and 23% of patients had pT1a and pT1b, respectively. Only 25% of patients with T1a tumors received systemic chemotherapy with or without trastuzumab.
The only phase II randomized trial in this review was the ATEMPT study published by Tarantino et al [30], in which patients with stage I HER2-positive tumors of ≤ 2 cm, with N0 or N1mi, were randomly assigned 3:1 to adjuvant T-DM1 for 1 year or paclitaxel plus trastuzumab (TH). In the T-DM1 arm, 16% and 33% of patients had T1a and T1b tumors, respectively, while the corresponding numbers in the TH arm were 17% and 35%, respectively.
Systematic analysis of data
In this review, statements about adjuvant therapy specify the use of adjuvant chemotherapy, trastuzumab/other anti-HER2 agents, or both without the effect of ET or radiotherapy. Table 2 shows the data on the number of patients offered and those who did not receive various adjuvant systemic therapy, and it also demonstrates the survival outcomes based on whether adjuvant therapy was offered.
 Click to view | Table 2. Frequency of Adjuvant Therapy Administration and the Corresponding Survival Outcomes |
Of the four prospective studies identified [22, 23, 25, 30], there were 510 patients with T1a tumors. In one study [22], the majority of patients (86.2%) received no adjuvant chemotherapy or trastuzumab; nevertheless, at 5 years, the reported DFS and distant disease-free survival (DDFS) in those patients was 97% and 99%, respectively. The corresponding rate for those who received chemotherapy with or without trastuzumab was 100% for either the DFS or OS.
In two prospective studies [25, 30], all patients received adjuvant chemotherapy plus trastuzumab. In the first of those studies, Dall et al reported a 5-year DFS of 96%. In the second study (ATEMPT), the reported 5-year invasive DFS was 97.7% and 90% for patients randomized to the T-DM1 and TH arms, respectively. Of note, only 20 patients in the TH arm had T1a tumors.
In the remaining prospective study with the largest number of patients (216) [23], patients who received chemotherapy with or without trastuzumab attained 100% 5-year DDFS and OS, regardless of their ER status. On the other hand, patients who did not receive adjuvant therapy and have ER+ demonstrated 5-year DDFS of 96% and OS of 95%, while among those with ER-, the 5-year DDFS and OS was 93% in each.
In the other two prospective studies, all patients received adjuvant chemotherapy plus trastuzumab [25, 30]. Despite the adjuvant therapy, Dall et al [25] reported a 5-year DFS of 96%, while the ATEMP trial [30] demonstrated a 97.7% invasive DFS for the T-DM1 randomized patients and 90% in 20 patients who received TH.
Of the nine retrospective studies, He et al [28], van Ramshorst et al [24], de Nonneville et al [26], and Kubo et al [29] reported on the largest numbers of patients with T1a tumors, 135, 138, 386, and 489, respectively. In He et al’s series, the 10-year DFS or OS was approximately 92% of the entire population of untreated (75%) and treated (25%) patients.
In the van Ramshorst et al’s data, there were 5% and 15% absolute 8-year breast cancer-specific survival and OS differences, respectively, favoring those who received chemotherapy with or without trastuzumab compared to those who received no adjuvant treatment. Nevertheless, those differences were not statistically significant.
Receiving chemotherapy and trastuzumab attained a 100% 5-year DFS for a small group of patients in the series reported by Gamucci et al [21]. On the other hand, in the series of de Nonneville et al [26], patients who received chemotherapy with or without trastuzumab attained a nonsignificant DFS rate compared to those who received no adjuvant therapy. Similarly, Kubo et al [29] reported 5-year DFS and OS of 96% and 99%, respectively, among the 75% of patients who did not receive adjuvant therapy, with no statistically significant survival difference between those who received treatment and the untreated group.
Meta-analysis
A meta-analysis was attempted by including studies that reported HR or allowed its estimate (Fig. 2), but only three studies were included in this analysis. The lack of sufficient data and relevant survival curves precluded using Tierney et al [13] and Parmar et al [14] methods to compute additional HRs from other studies. The analysis showed a survival trend in favor of the treated group compared to those who received no adjuvant therapy; however, the analysis showed significant heterogeneity (I2 > 50%). The lack of sufficient data about potential confounders, such as tumor grade or ER status, precluded performing a meta-regression to investigate such heterogeneity.
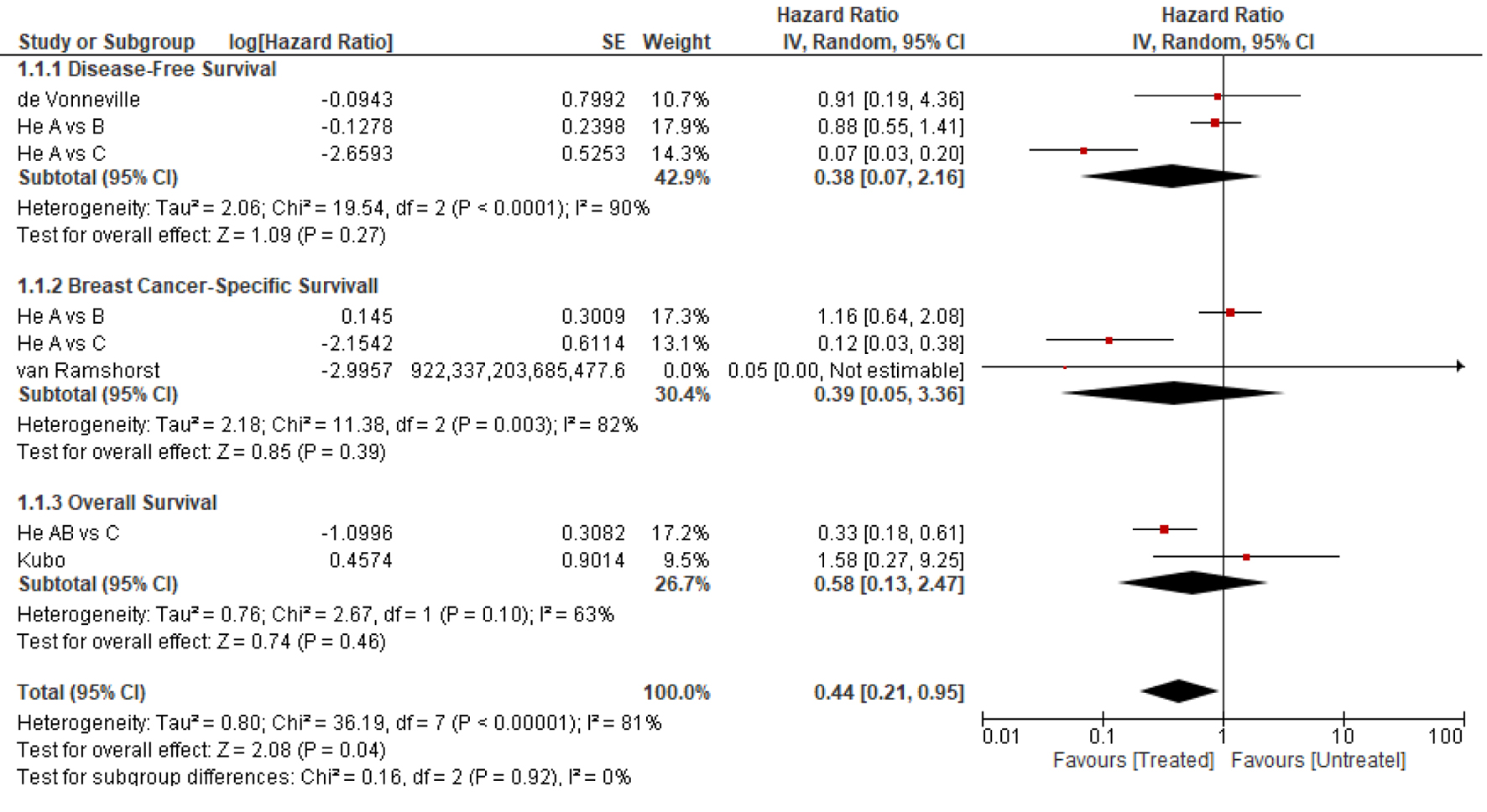 Click for large image | Figure 2. The forest plot of the pooled hazard ratio for survival outcomes (random-effect model). All statistical tests were two-sided. He A: group of patients who did not receive adjuvant therapy; He B: group of patients who received chemotherapy only; He C: group of patients who received chemotherapy and trastuzumab. |
Defining prognostic factors
Two studies reported the influence of ER status on survival outcomes for patients with T1a tumors. Curigliano et al [19] showed that patients with ER+ demonstrated an inferior 5-year DFS (88%) compared to those with ER- tumors (93%). In their series, 53% of patients with T1a-b had ER+ tumors. In the study by Vaz-Luis et al [23], 62% of patients with T1a had ER+ tumors. The authors reported that patients with ER+ disease attained a nonsignificant 2-3% DDFS and OS advantage compared to those with ER- tumors.
There were insufficient data to examine the effect of other variables, such as tumor size (1 - 5 cm), tumor grade, or Ki-67, on patient outcomes.
| Discussion | ▴Top |
The review highlights the impact of adjuvant therapy, specifically chemotherapy, trastuzumab, or both, on survival outcomes in patients with HER2-positive T1aN0M0 breast tumors. The data from both prospective and retrospective studies provide a thorough overview of the possible efficacy of these treatments.
The prospective studies included in the review provide important information concerning the survival outcomes of patients with T1a tumors who all received adjuvant therapy. Notably, Dall et al [25] reported high 5-year DFS rates of 99% among patients given adjuvant chemotherapy plus trastuzumab. In the ATEMPT trail [30], while the 5-year invasive DFS for patients who received T-DM1 was favorable (97.7%), those assigned to the TH arm had a worse invasive DFS rate of 90%. In the largest prospective study [23], patients who received adjuvant chemotherapy with or without trastuzumab attained an absolute survival difference of 4-7% regardless of the ER compared to those without adjuvant treatment. These findings indicate that adjuvant therapy can provide a small but meaningful survival benefit, particularly in high-risk patients.
The data also imply that many patients with T1a tumors would have favorable outcomes without adjuvant therapy. The retrospective studies supported this assessment. In He et al’s series [28], where 75% were untreated, the 10-year DFS or OS was approximately 92%. Furthermore, in the study by Kubo et al [29], two-thirds of patients who did not receive adjuvant treatment achieved a 5-year DFS and OS of 96% and 99%, respectively. The lack of a significant survival difference between treated groups was also found in the series of de Nonneville et al [26]. Nevertheless, van Ramshorst et al [24] reported a trend for patients who received chemotherapy with or without trastuzumab to achieve better survival, although the differences were not statistically significant.
The additional cost of combining chemotherapy plus trastuzumab versus chemotherapy alone would only be justified if it were associated with a survival benefit. Unfortunately, this question could not be assessed as only one study [22] reported the outcomes of patients who received chemotherapy only and those who received chemotherapy plus trastuzumab. The number of patients was small, 9 and 6, respectively. In both groups, the locoregional-free survival, the DFS, and the OS at 5 years were 100% in each group. Therefore, there were not enough data to draw any valuable conclusions.
In the meta-analysis, a survival trend favored the treated group compared to those who received no adjuvant therapy; however, only three studies were included. Furthermore, the analysis showed significant heterogeneity that could not be investigated due to the lack of data on the known confounders.
Effect of ER status
It is known that possible crosstalk between HER2 and ER results in intrinsic and acquired resistance to ET and a poorer prognosis [31]. In this review, we have identified some inconsistencies.
Curigliano et al [19] reported a worse prognosis among patients who had T1a ER+ tumors than those who had ER- tumors. Conversely, the authors found that patients with T1b ER+ tumors had a 10% survival advantage compared with those with ER- (95% vs. 85%). Rouanet et al [32] concurred with Curigliano et al’s conclusion about the negative impact of co-expression of ER+ tumors. They showed that patients with ER+ T1ab tumors had a worse DFS than those who harbored HR- tumors (65% vs. 82%). On the other hand, regardless of the HR status, Kubo et al [27] showed no significant treatment effect on OS or DFS.
Caution should be exerted concerning conclusions about the patient outcome as influenced by the co-existing ER status, particularly that none of the studies have reported the compliance rate to ET therapy; moreover, in some studies, not all patients with ER+ tumors received adjuvant ET [22].
The most significant evidence that adjuvant therapy in early HER2-positive breast cancer could have mitigated the prognostic significance for the ER status comes from the prospective study of Tolaney et al [33], which showed that for all stage I patients who received adjuvant TH therapy, the 5-year invasive DFS was similar in ER+ (96.8%) and ER- (97.6%) patients.
Limitations
Although this research represents the only systematic review and meta-analysis that examined data exclusive to patients with T1a tumors, we acknowledge several limitations.
First, variability in treatment as most studies, such as Vaz-Luos et al [23] and Fehrenbacher et al [22], had variations in chemotherapy and trastuzumab administration rates over time and may have introduced patient and physician bias. Second, the study design, as of the 11 included studies, only four were prospective trials, and the only randomized trial was designed as a single-arm trial [30]. Third, the number of patients reviewed in the included studies varied significantly. Therefore, one should exercise caution in overinterpreting the findings of small-sample studies. Fourth, in this review, we could not ascertain the prognostic effect of the ER status; furthermore, the lack of sufficient data precluded the evaluation of other prognostic variables such as tumor size (1 - 5 cm), tumor grade, and Ki-67 expression. Fifth, the included studies did not report data on the impact of loss to follow-up, nor did they report the loss to follow-up rate. Sixth, while two studies reported survival data at approximately 10 years [18, 32], the reported median follow-up for the other trials was relatively short and may not capture all expected recurrences, particularly in patients with hormone ER+ tumors. Finally, quality-of-life and treatment toxicity data were lacking except for the only prospective, randomized trial [28].
Conclusion
The review underscores the complexity of treatment decisions for patients with T1a breast tumors. While adjuvant chemotherapy and trastuzumab can improve survival outcomes, a substantial proportion of patients may achieve excellent survival without these treatments. Despite the trend favoring adjuvant therapy, no significant survival benefit was demonstrated. Therefore, the decision to prescribe adjuvant therapy should be individualized according to the balance between the expected risks and benefits. Furthermore, patient preference studies have strongly suggested that many women may consider a moderately toxic treatment for a relatively small benefit. Our results also suggest the need for caution to avoid overtreatment and to consider drug toxicity and financial toxicity and also highlight the importance of using the less toxic regimens in the setting of small predicted absolute benefits [30, 33]. Moreover, considering the marginal benefit, future trials that would include such patients should assess the impact of adjuvant interventions on the quality of life.
Acknowledgments
None to declare.
Financial Disclosure
For this research, we received no funding from any source.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Prof. Ezzeldin Ibrahim: conceptualization, data collection, data analysis, administrative role, software providing, manuscript writing, approval of the manuscript, and manuscript submission. Prof. Ahmed Refae, Dr. Ali Bayer, Dr. Nouf Abdullah, and Dr. Meteb Al-Foheidi: conceptualization, data collection, administrative role, manuscript writing, and approval of the manuscript.
Data Availability
The project was uploaded for public access to the OSF database repository: osf.io/2y3xg. The project was uploaded for public access to the Figshare database repository: figshare - credit for all your research - <b>Adjuvant therapy benefit for patients with HER2-positive T1aN0M0 breast cancer: a systemic review and meta-analysis</b> - Item Edit.
| References | ▴Top |
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784-1792.
doi pubmed - Kennedy T, Stewart AK, Bilimoria KY, Patel-Parekh L, Sener SF, Winchester DP. Treatment trends and factors associated with survival in T1aN0 and T1bN0 breast cancer patients. Ann Surg Oncol. 2007;14(10):2918-2927.
doi pubmed - Perry N, Broeders M, de Wolf C, Tornberg S, Holland R, von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition—summary document. Ann Oncol. 2008;19(4):614-622.
doi pubmed - Khan M, Chollet A. Breast cancer screening: common questions and answers. Am Fam Physician. 2021;103(1):33-41.
pubmed - Ahn S, Woo JW, Lee K, Park SY. HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. J Pathol Transl Med. 2020;54(1):34-44.
doi pubmed - Kelly CM, Pritchard KI, Trudeau M, Andreopoulou E, Hess K, Pusztai L. Coping with uncertainty: T1a,bN0M0 HER2-positive breast cancer, do we have a treatment threshold? Ann Oncol. 2011;22(11):2387-2393.
doi pubmed - Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G, Jr., et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195-1205.
doi pubmed - Piccart M, Procter M, Fumagalli D, de Azambuja E, Clark E, Ewer MS, Restuccia E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years' follow-up. J Clin Oncol. 2021;39(13):1448-1457.
doi pubmed - Early Breast Cancer Trialists’ Collaborative group (EBCTCG). Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021;22(8):1139-1150.
doi pubmed - Oncology NCCPG. National Comprehensive Cancer Network Clinical Practice Guidelines Oncology. 2024.
- Paluch-Shimon S, Cardoso F, Partridge AH, Abulkhair O, Azim HA, Bianchi-Micheli G, Cardoso MJ, et al. ESO-ESMO fifth international consensus guidelines for breast cancer in young women (BCY5). Ann Oncol. 2022;33(11):1097-1118.
doi pubmed - Hassing CMS, Nielsen DL, Knoop AS, Tvedskov THF, Kroman N, Laenkholm AV, Juhl CB, et al. Adjuvant treatment with trastuzumab of patients with HER2-positive, T1a-bN0M0 breast tumors: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2023;184:103952.
doi pubmed - Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
doi pubmed - Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815-2834.
doi pubmed - DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
doi pubmed - Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558.
doi pubmed - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed - Joensuu H, Isola J, Lundin M, Salminen T, Holli K, Kataja V, Pylkkanen L, et al. Amplification of erbB2 and erbB2 expression are superior to estrogen receptor status as risk factors for distant recurrence in pT1N0M0 breast cancer: a nationwide population-based study. Clin Cancer Res. 2003;9(3):923-930.
pubmed - Curigliano G, Viale G, Bagnardi V, Fumagalli L, Locatelli M, Rotmensz N, Ghisini R, et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009;27(34):5693-5699.
doi pubmed - Livi L, Meattini I, Saieva C, Franzese C, Di Cataldo V, Greto D, Franceschini D, et al. Prognostic value of positive human epidermal growth factor receptor 2 status and negative hormone status in patients with T1a/T1b, lymph node-negative breast cancer. Cancer. 2012;118(13):3236-3243.
doi pubmed - Gamucci T, Vaccaro A, Ciancola F, Pizzuti L, Sperduti I, Moscetti L, Longo F, et al. Recurrence risk in small, node-negative, early breast cancer: a multicenter retrospective analysis. J Cancer Res Clin Oncol. 2013;139(5):853-860.
doi pubmed - Fehrenbacher L, Capra AM, Quesenberry CP, Jr., Fulton R, Shiraz P, Habel LA. Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: a cohort from an integrated health care delivery system. J Clin Oncol. 2014;32(20):2151-2158.
doi pubmed - Vaz-Luis I, Ottesen RA, Hughes ME, Mamet R, Burstein HJ, Edge SB, Gonzalez-Angulo AM, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol. 2014;32(20):2142-2150.
doi pubmed - van Ramshorst MS, van der Heiden-van der Loo M, Dackus GM, Linn SC, Sonke GS. The effect of trastuzumab-based chemotherapy in small node-negative HER2-positive breast cancer. Breast Cancer Res Treat. 2016;158(2):361-371.
doi pubmed - Dall P, Koch T, Gohler T, Selbach J, Ammon A, Eggert J, Gazawi N, et al. Trastuzumab in human epidermal growth factor receptor 2-positive early breast cancer: results of a prospective, noninterventional study on routine treatment between 2006 and 2012 in Germany. Oncologist. 2017;22(2):131-138.
doi pubmed - de Nonneville A, Goncalves A, Zemmour C, Classe JM, Cohen M, Lambaudie E, Reyal F, et al. Benefit of adjuvant chemotherapy with or without trastuzumab in pT1ab node-negative human epidermal growth factor receptor 2-positive breast carcinomas: results of a national multi-institutional study. Breast Cancer Res Treat. 2017;162(2):307-316.
doi pubmed - Musolino A, Falcini F, Sikokis A, Boggiani D, Rimanti A, Pellegrino B, Silini EM, et al. Prognostic impact of interval breast cancer detection in women with pT1a N0M0 breast cancer with HER2-positive status: Results from a multicentre population-based cancer registry study. Eur J Cancer. 2018;88:10-20.
doi pubmed - He X, Ji J, Tian M, Esteva FJ, Hortobagyi GN, Yeung SJ. Long-term survival analysis of adjuvant chemotherapy with or without trastuzumab in patients with T1, node-negative HER2-positive breast cancer. Clin Cancer Res. 2019;25(24):7388-7395.
doi pubmed - Kubo M, Kawai M, Kumamaru H, Miyata H, Tamura K, Yoshida M, Ogo E, et al. A population-based recurrence risk management study of patients with pT1 node-negative HER2+ breast cancer: a National Clinical Database study. Breast Cancer Res Treat. 2019;178(3):647-656.
doi pubmed - Tarantino P, Tayob N, Villacampa G, Dang C, Yardley DA, Isakoff SJ, Valero V, et al. Adjuvant trastuzumab emtansine versus paclitaxel plus trastuzumab for stage I human epidermal growth factor receptor 2-positive breast cancer: 5-year results and correlative analyses from ATEMPT. J Clin Oncol. 2024;42(31):3652-3665.
doi pubmed - De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, Tortora G, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11(13):4741-4748.
doi pubmed - Rouanet P, Roger P, Rousseau E, Thibault S, Romieu G, Mathieu A, Cretin J, et al. HER2 overexpression a major risk factor for recurrence in pT1a-bN0M0 breast cancer: results from a French regional cohort. Cancer Med. 2014;3(1):134-142.
doi pubmed - Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, Albain KS, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134-141.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.