| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 3, June 2025, pages 269-275
Prognostic Significance of Post-Neoadjuvant Chemotherapy Carbohydrate Antigen 19-9 Levels in Patients With Resectable Pancreatic Cancer Treated With S-1 and Gemcitabine: A Retrospective Cohort Study
Yuki Hommaa, b , Kentaro Miyakea, Yutaro Kikuchia, Yasuhiro Yabushitaa, Ryusei Matsuyamaa, Itaru Endoa
aDepartment of Gastroenterological Surgery, Yokohama City University School of Medicine, Yokohama City, Japan
bCorresponding Author: Yuki Homma, Department of Gastroenterological Surgery, Yokohama City University School of Medicine, Kanazawa-ku, Yokohama City, Japan
Manuscript submitted February 22, 2025, accepted May 22, 2025, published online June 14, 2025
Short title: Prognostic Value of CA19-9 in Pancreatic Cancer
doi: https://doi.org/10.14740/wjon2563
| Abstract | ▴Top |
Background: Carbohydrate antigen 19-9 (CA19-9) is widely used to assess treatment response and monitor recurrence alongside imaging. However, the criteria for determining resectability after completion of neoadjuvant therapy (NAT) remain poorly defined. Therefore, this study aimed to investigate the indications for surgical resection as a prognostic factor following NAT with gemcitabine and S-1 (NATGS).
Methods: In this retrospective cohort study, we examined patients who underwent curative pancreatic resection following NATGS at our institution between April 2018 and December 2023. After excluding six patients who did not undergo pancreatectomy, the remaining 50 patients were included in the study. Univariate and multivariate analyses were conducted to identify factors potentially associated with survival after NATGS.
Results: Post-NATGS CA19-9 levels (< 100 U/mL) were identified as a significant prognostic factor for disease-free survival (DFS) in both univariate and multivariate analyses (hazard ratio (HR) = 11.72251, P < 0.001). For overall survival (OS), both CA19-9 levels (< 100 U/mL) and Duke pancreatic monoclonal antigen type 2 (DUPAN-2) levels (< 150 U/mL) were significant prognostic factors in univariate and multivariate analyses (CA19-9: HR = 17.88, P = 0.002; DUPAN-2: HR = 2.667, P = 0.03). The median DFS was 24.1 months in the low CA19-9 group compared with the 7.1 months in the high CA19-9 group (P = 0.002). The median OS in the low CA19-9 group was not reached, whereas it was 14.7 months in the high CA19-9 group (P = 0.001).
Conclusions: The CA19-9 cut-off value is clinically significant for patients undergoing NATGS regimens. Patients with pre-operative CA19-9 levels ≥ 100 U/mL may benefit from extended GS treatment or a switch to a more potent regimen rather than proceeding directly to surgical resection.
Keywords: Pancreatic cancer; CA19-9; Neoadjuvant therapy; NATGS
| Introduction | ▴Top |
Pancreatic ductal adenocarcinoma (PDAC) is associated with a poor prognosis, with frequent early recurrence following surgery due to its aggressive nature. The number of deaths due to pancreatic cancer is increasing in Japan and the USA [1, 2].
Surgery alone is insufficient to achieve favorable survival outcomes [3]. Advancements in neoadjuvant and post-operative adjuvant treatments have contributed to improved survival rates following pancreatic resection for PDAC. The potential benefits of neoadjuvant chemotherapy (NAC) include reducing tumor burden, which may facilitate curative resection, and enabling the early administration of systemic treatment for micrometastases that are not detectable on radiological imaging [4].
For patients with anatomically resectable pancreatic cancer, a randomized phase III trial (Prep-02/JSAP-05) conducted in Japan reported an improved prognosis with two cycles of NATGS therapy. Based on these findings, the Japanese clinical practice guidelines recommend combination neoadjuvant therapy (NAT) with gemcitabine (GEM) plus S-1 (NATGS) as a standard neoadjuvant approach. Consequently, NATGS has been widely adopted in Japan for the treatment of anatomically resectable pancreatic cancer, regardless of carbohydrate antigen 19-9 (CA19-9) levels. However, the factors that determine patient suitability for resection following NATGS remain largely unexplored [5, 6].
CA19-9 is a diallylated Lewis blood group antigen, which was first identified as a tumor marker in 1981 [7]. Elevated CA19-9 levels are a well-established prognostic biomarker for pancreatic cancer. In addition to imaging, CA19-9 is primarily used to assess treatment response and monitor recurrence [8-10]. The 2019 American Society of Clinical Oncology (ASCO) [11] and 2022 National Comprehensive Cancer Network (NCCN) guidelines recommend upfront surgery for resectable pancreatic cancer and neoadjuvant therapy for borderline resectable pancreatic cancer [12]. Furthermore, these ASCO and NCCN guidelines suggest considering neoadjuvant therapy for patients with resectable pancreatic cancer who have “markedly elevated” CA19-9 levels. However, the predictive cut-off value of CA19-9 for selecting patients who would benefit from neoadjuvant therapy remains insufficiently studied. Additionally, re-evaluating CA19-9 levels after completion of NATGS is crucial for further clinical decision-making.
The criteria for determining resectability after the completion of NATGS are not well defined. Therefore, this study aimed to investigate the prognostic factors (CA19-9) that serve as indications for resection following the completion of NATGS in patients with anatomical resectable pancreatic cancer.
| Materials and Methods | ▴Top |
Patients
This retrospective cohort study enrolled 56 patients diagnosed with anatomically resectable PDAC, as defined by the Japan Pancreatic Society (JPS), at the time of initial diagnosis. The patients underwent curative pancreatic resection following NATGS at our institution between April 2018 and December 2023. After excluding six patients who did not undergo pancreatectomy, a total of 50 patients were included in the analysis. The collected data were retrospectively examined in accordance with investigational protocols approved by the Institutional Review Board and Ethics Committee of Yokohama City University (approval number B180600049). The study was conducted in compliance with the ethical standards outlined in the Declaration of Helsinki. Informed consent was obtained using the opt-out principle.
Pre-operative management and neoadjuvant therapy
The resectability of pancreatic cancer was assessed before the initiation of NATGS using thin-slice enhanced abdominal computed tomography, as defined by the JPS. The NATGS regimen consisted of GEM plus S-1. Specifically, the GEM plus S-1 regimen included 1,000 mg/m2 GEM administered intravenously on days 1 and 8, combined with 60 mg/m2 oral S-1 administered on days 1 - 14. Patients’ disease was clinically and radiographically restaged within 4 - 6 weeks of completing NATGS. Tumor markers, including carcinoembryonic antigen (CEA), CA19-9, and Duke pancreatic monoclonal antigen type 2 (DUPAN-2), were measured both before and after NATGS. CA19-9 levels were considered reliable only when total bilirubin levels were < 2.0 U/mL.
Patients with no evidence of disease progression on imaging studies and who had an adequate performance status were considered for surgical resection and underwent pancreatoduodenectomy, distal pancreatectomy, or total pancreatectomy. For all patients who recovered from surgery, we recommended administering adjuvant chemotherapy using S-1 for 6 months.
Statistical analyses
Statistical analyses were performed using JMP Pro® version 19 (SAS Institute Inc., Cary, NC, USA). The cut-off values for CA19-9 and DUPAN-2 were 100 and 150 U/mL, respectively. To determine the optimal cut-off for CA19-9 and DUPAN-2 in predicting recurrence-free survival in pancreatic cancer, we used R (version 4.0.0) with the survival and survminer packages. A Cox proportional hazards model was applied to assess the impact of CA19-9 and DUPAN-2 on survival. The optimal cut-off value was determined by maximizing the hazard ratio (HR) using various cut-off points, evaluated via Kaplan-Meier survival analysis and the surv_cutpoint function. Patients were classified into high-risk (CA19-9 or DUPAN2 ≥ cut-off) and low-risk (CA19-9 or DUPAN-2 < cut-off) groups. The other factor, cut-off values were chosen on median values. The patients were divided into groups based on their low and high CA19-9 levels. Differences between subgroups for continuous variables were analyzed using the nonparametric Student’s t-test. Fisher’s exact test was used to analyze categorical variables. Survival rates were estimated using the Kaplan-Meier method, and the log-rank test was used to compare survival between the two groups. Univariate and multivariate proportional hazard regression analyses were performed to assess parameters potentially associated with survival after NATGS. Two-sided P-values were considered statistically significant at P ≤ 0.05.
| Results | ▴Top |
Patient characteristics
Between April 2018 and March 2023, data were collected from 56 patients with resectable pancreatic cancer treated with NATGS. Pancreatectomy was performed in 50 of these 56 patients. The six patients who did not undergo resection included one with liver metastasis, one with para-aortic lymph node metastasis, one with local progression, and three who declined surgery.
This study included 30 male and 20 female patients, with a median age of 72 years. We compared the low and high CA19-9 groups after the completion of NATGS. Significant differences were observed in the pre- and post-NATGS CA19-9 and DUPAN-2 levels. Pathological tumor size did not differ significantly, and the rate of nodal metastasis was higher in the high CA19-9 group, although the difference was not statistically significant. The incidence of adverse events and the completion rate of the GS regimen for two cycles were similar between the groups, and the completion rate of adjuvant chemotherapy using S1 was also the same in this cohort (Table 1).
 Click to view | Table 1. Descriptive Statistics for the Total Cohort and Two Subgroups Stratified by Post-NATGS CA19-9 Levels |
Prognostic factors after NATGS
Univariate and multivariate Cox regression analyses were performed to evaluate the effect of these factors on disease-free survival (DFS) and overall survival (OS). DFS was defined as the time from pancreatectomy to the first confirmation of recurrence, and OS was defined as the time from initiation of NAC to death. All pre-operative factors used in the study were measured after completing the NATGS regimen. Univariate and multivariate analyses of prognostic factors for DFS are presented in Table 2. CA19-9 levels (pre-NATGS) were significant in univariate analysis (P = 0.039), but not in multivariate analysis. Only CA19-9 levels (post-NATGS) were identified as significant prognostic factors in both univariate and multivariate analyses (HR = 11.72251, P < 0.001). For OS, CA19-9 (post-NATGS) and DUPAN-2 (post-NATGS) levels were identified as prognostic factors in both univariate and multivariate analyses (CA19-9: HR = 17.88, P = 0.002; DUPAN-2: HR = 2.667, P = 0.03) (Table 3).
 Click to view | Table 2. Univariate and Multivariate Analysis of Predictive Factors for Disease-Free Survival |
 Click to view | Table 3. Univariate and Multivariate Analysis of Predictive Factors for Overall Survival |
The Kaplan-Meier curves for DFS and OS are shown in Figure 1. The low CA19-9 (post-NATGS) group showed a significantly better prognosis in terms of both DFS and OS after NAT (median DFS: favorable vs. unfavorable = 24.1 months vs. 7.1 months, P = 0.002; median OS: favorable vs. unfavorable = not reached vs. 14.7 months, P = 0.001).
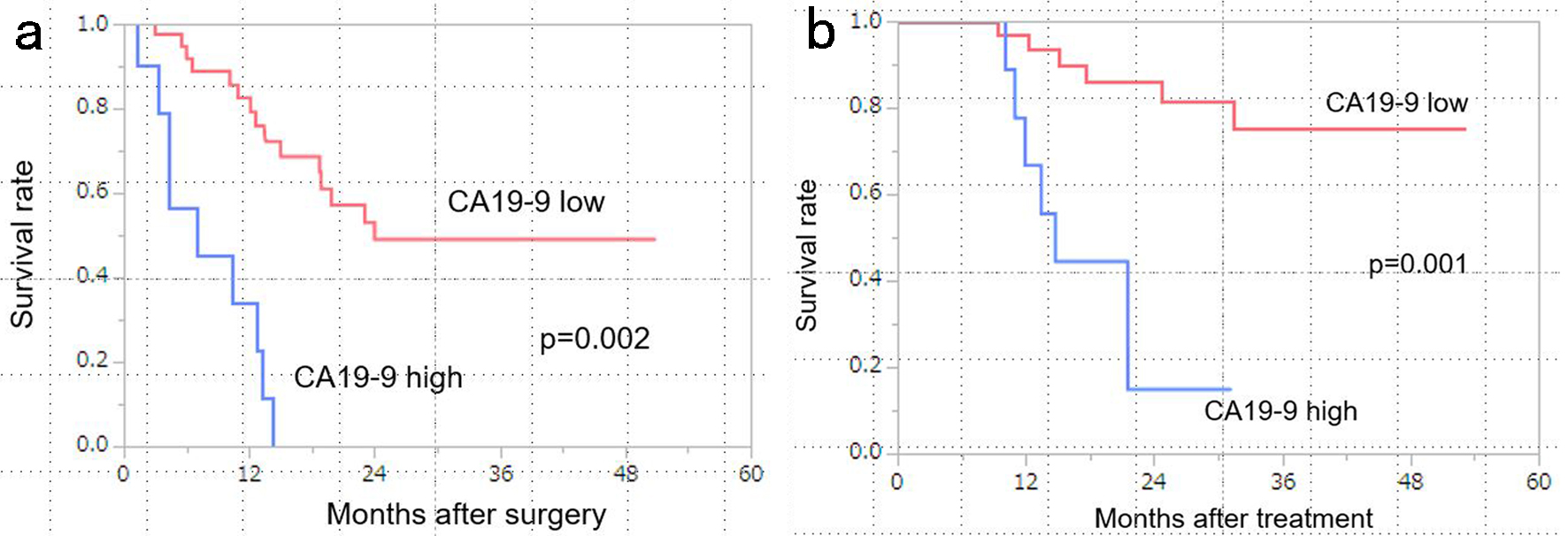 Click for large image | Figure 1. Kaplan-Meier curves stratified by CA19-9 levels (low vs. high). (a) Patients with low CA19-9 levels (< 100 U/mL) had significantly better disease-free survival (DFS) than those with high CA19-9 levels (median survival time (MST): low CA19-9, 24 months vs. high CA19-9, 7.0 months; P = 0.002). (b) Patients with low CA19-9 levels (< 100 U/mL) had significantly better overall survival (OS) than those with high CA19-9 levels (MST: low CA19-9, not reached vs. high CA19-9, 14.7 months; P = 0.001). CA19-9: carbohydrate antigen 19-9. |
Pattern of initial recurrence
In the entire cohort, liver metastases were the most commonly observed recurrence pattern (42%), followed by peritoneal metastases (38%) and locoregional metastases, including those in the remnant pancreas (21%). Regarding the site of recurrence, no significant difference was observed between the low and high CA19-9 groups. For post-relapse treatment, 10 of 11 patients (91%) in the low CA19-9 group and eight of nine patients (88%) in the high CA19-9 group were treated with chemotherapy.
| Discussion | ▴Top |
This study investigated the prognostic significance of post-NAT CA19-9 levels in patients with resectable pancreatic cancer who were treated with NATGS. The findings demonstrated that patients with lower CA19-9 levels (cut-off value: CA19-9 < 100 U/mL) after NATGS treatment had a better prognosis. The main benefits of pre-operative therapy include: 1) reduced risk of potential distant metastases; 2) introduction of chemotherapy in good pre-operative general condition; 3) improved resection rates for curative surgery; 4) reduced tumor size and lymph node metastasis rates; 5) identification of patients whose disease is rapidly deteriorating and therefore would not benefit from surgery; and 6) improved OS. Disadvantages include the risk of tumor growth, deterioration of general condition due to chemotherapy, and the risk of overtreatment [4].
In Japan, pre-operative treatment with the GS regimen for anatomically resectable pancreatic cancer has been included in the guidelines since the PREP02 trial and has become a common treatment option. While some patients benefit from this regimen, others experience early recurrence, and the clinical data of patients who benefit from surgery after GS NAT remain unclear [5, 6].
CA19-9 levels have been reported as prognostic factors in pancreatic cancer [13, 14]. Tsai et al reported that normalization of CA19-9 levels after NAT is a prognostic factor in patients with resectable or borderline resectable tumors [15]. Aoki et al also reported that patients with low CA19-9 levels (< 103 U/mL) after NAT had a good prognosis [16]. These studies used multiple regimens rather than a single regimen, whereas this study was a single regimen study.
The non-clinical responder group had worse clinical outcomes. These patients may require a more potent change in the therapy regimen. Treatments more effective than GS include gemcitabine, nab-paclitaxel (GnP), and FOLFIRINOX. Ikenaga et al reported the efficacy of pre-operative GnP therapy for borderline resectable (BR) pancreatic cancer [17], and the PREOPANC-2 trial using FOLFIRINOX as the NAT showed favorable results [18, 19]. However, these potent regimens are not suitable for all patients as pre-operative treatments and should be reserved for those with poor prognoses.
Isaji et al proposed a classification system for biological resectability. Even in anatomically resectable pancreatic cancer, a high CA19-9 level is associated with a poor prognosis, especially if the CA19-9 level is above 500 U/mL, which is defined as the biological borderline [20]. Alva-Ruiz et al reported an improved prognosis for locally advanced pancreatic cancer when the pre-operative treatment regimen was modified (switch therapy) according to the patient’s biological borderline status [21].
The study results here suggest that patients who underwent two cycles of NATGS for anatomically resectable pancreatic cancer and achieved a partial response or stable disease on imaging but had CA19-9 levels > 100 U/mL after NATGS had a poor prognosis. The CA19-9 level before NATGS was significant in univariate analysis of DFS, but not in multivariate analysis, suggesting that CA19-9 after NATGS is a more useful factor in making a resection decision. Moreover, patients with markedly reduced CA19-9 levels but still high levels (> 100 U/mL) may require several additional courses of GS therapy before surgery, which could result in a higher survival rate. Additionally, for patients whose CA19-9 levels do not decrease and remain high, it may be necessary to switch to FOLFIRINOX or GnP therapy.
This study has some limitations. First, GS therapy is widely used only in Japan and is rarely practiced in Europe or the USA. Second, the follow-up period was short, and the number of patients was small. Furthermore, only six patients’ CA19-9 levels decreased from > 100 to < 100 U/mL after NATGS. Therefore, the effect of biological conversion on improving prognosis could not be fully examined. However, we report that the pre-operative CA19-9 level after NATGS is an important prognostic factor for the GS regimen recommended in Japan.
In conclusion, this study demonstrated that the CA19-9 cut-off value is clinically important for patients undergoing NAT with GS regimens. A pre-operative CA19-9 level > 100 U/mL indicates the need for prolonged GS treatment or a switch to a more potent regimen, rather than proceeding with resection.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained using the opt-out principle.
Author Contributions
YH and KM contributed to the study design. All the authors contributed to the data collection and interpretation. All the authors contributed to the writing or review of the report and approved the final version.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ASCO: American Society of Clinical Oncology; CA19-9: carbohydrate antigen 19-9; DFS: disease-free survival; GEM: gemcitabine; GnP: gemcitabine plus nab-paclitaxel; GS: gemcitabine plus S-1; HR: hazard ratio; JPS: Japan Pancreatic Society; NAT: neoadjuvant therapy; NATGS: neoadjuvant therapy with gemcitabine plus S-1; NCCN: National Comprehensive Cancer Network; OS: overall survival; PDAC: pancreatic ductal adenocarcinoma
| References | ▴Top |
- Chen X, Yi B, Liu Z, Zou H, Zhou J, Zhang Z, Xiong L, et al. Global, regional and national burden of pancreatic cancer, 1990 to 2017: Results from the Global Burden of Disease Study 2017. Pancreatology. 2020;20(3):462-469.
doi pubmed - Higashi T, Kurokawa Y. Incidence, mortality, survival, and treatment statistics of cancers in digestive organs-Japanese cancer statistics 2024. Ann Gastroenterol Surg. 2024;8(6):958-965.
doi pubmed - Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008-2020.
doi pubmed - Lambert A, Schwarz L, Ducreux M, Conroy T. Neoadjuvant treatment strategies in resectable pancreatic cancer. Cancers (Basel). 2021;13(18):4724.
doi pubmed - Unno M, Motoi F, Matsuyama Y, Satoi S, Matsumoto I, Aosasa S, Shirakawa H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J Clin Oncol. 2019;37(4_suppl):189.
doi - Satoi S, Unnno M, Moti F, Matsuyama Y, Matsumoto I, Aosasa S, Shirakawa H, et al. The effect of neoadjuvant chemotherapy with gemcitabine and S-1 for resectable pancreatic cancer (randomized phase II/III trial: Prep-02/JSAP-05). J Clin Oncol. 2019;37(suppl 15):4126.
doi - Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212(4490):53-55.
doi pubmed - Murakawa M, Kawahara S, Takahashi D, Kamioka Y, Yamamoto N, Kobayashi S, Ueno M, et al. Risk factors for early recurrence in patients with pancreatic ductal adenocarcinoma who underwent curative resection. World J Surg Oncol. 2023;21(1):263.
doi pubmed - Murata Y, Ogura T, Hayasaki A, Gyoten K, Ito T, Iizawa Y, Fujii T, et al. Predictive risk factors for early recurrence in patients with localized pancreatic ductal adenocarcinoma who underwent curative-intent resection after preoperative chemoradiotherapy. PLoS One. 2022;17(4):e0264573.
doi pubmed - Takahashi H, Yamada D, Asukai K, Wada H, Hasegawa S, Hara H, Shinno N, et al. Clinical implications of the serum CA19-9 level in "biological borderline resectability" and "biological downstaging" in the setting of preoperative chemoradiation therapy for pancreatic cancer. Pancreatology. 2020;20(5):919-928.
doi pubmed - Khorana AA, McKernin SE, Berlin J, Hong TS, Maitra A, Moravek C, Mumber M, et al. Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update. J Clin Oncol. 2019;37(23):2082-2088.
doi pubmed - NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma Version 1.2025: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
- Yamada S, Hashimoto D, Yamamoto T, Yamaki S, Oshima K, Murotani K, Sekimoto M, et al. Reconsideration of the clinical impact of neoadjuvant therapy in resectable and borderline resectable pancreatic cancer: A dual-institution collaborative clinical study. Pancreatology. 2024;24(4):592-599.
doi pubmed - Perri G, Prakash L, Wang H, Bhosale P, Varadhachary GR, Wolff R, Fogelman D, et al. Radiographic and serologic predictors of pathologic major response to preoperative therapy for pancreatic cancer. Ann Surg. 2021;273(4):806-813.
doi pubmed - Tsai S, George B, Wittmann D, Ritch PS, Krepline AN, Aldakkak M, Barnes CA, et al. Importance of normalization of CA19-9 levels following neoadjuvant therapy in patients with localized pancreatic cancer. Ann Surg. 2020;271(4):740-747.
doi pubmed - Aoki S, Motoi F, Murakami Y, Sho M, Satoi S, Honda G, Uemura K, et al. Decreased serum carbohydrate antigen 19-9 levels after neoadjuvant therapy predict a better prognosis for patients with pancreatic adenocarcinoma: a multicenter case-control study of 240 patients. BMC Cancer. 2019;19(1):252.
doi pubmed - Ikenaga N, Miyasaka Y, Ohtsuka T, Nakata K, Adachi T, Eguchi S, Nishihara K, et al. A prospective multicenter phase II trial of neoadjuvant chemotherapy with gemcitabine plus nab-paclitaxel for borderline resectable pancreatic cancer with arterial involvement. Ann Surg Oncol. 2023;30(1):193-202.
doi pubmed - Janssen QP, van Dam JL, Bonsing BA, Bos H, Bosscha KP, Coene P, van Eijck CHJ, et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer. 2021;21(1):300.
doi pubmed - van Dam JL, Verkolf EMM, Dekker EN, Bonsing BA, Bratlie SO, Brosens LAA, Busch OR, et al. Perioperative or adjuvant mFOLFIRINOX for resectable pancreatic cancer (PREOPANC-3): study protocol for a multicenter randomized controlled trial. BMC Cancer. 2023;23(1):728.
doi pubmed - Isaji S, Mizuno S, Windsor JA, Bassi C, Fernandez-Del Castillo C, Hackert T, Hayasaki A, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2-11.
doi pubmed - Alva-Ruiz R, Yohanathan L, Yonkus JA, Abdelrahman AM, Gregory LA, Halfdanarson TR, Mahipal A, et al. Neoadjuvant chemotherapy switch in borderline resectable/locally advanced pancreatic cancer. Ann Surg Oncol. 2022;29(3):1579-1591.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.