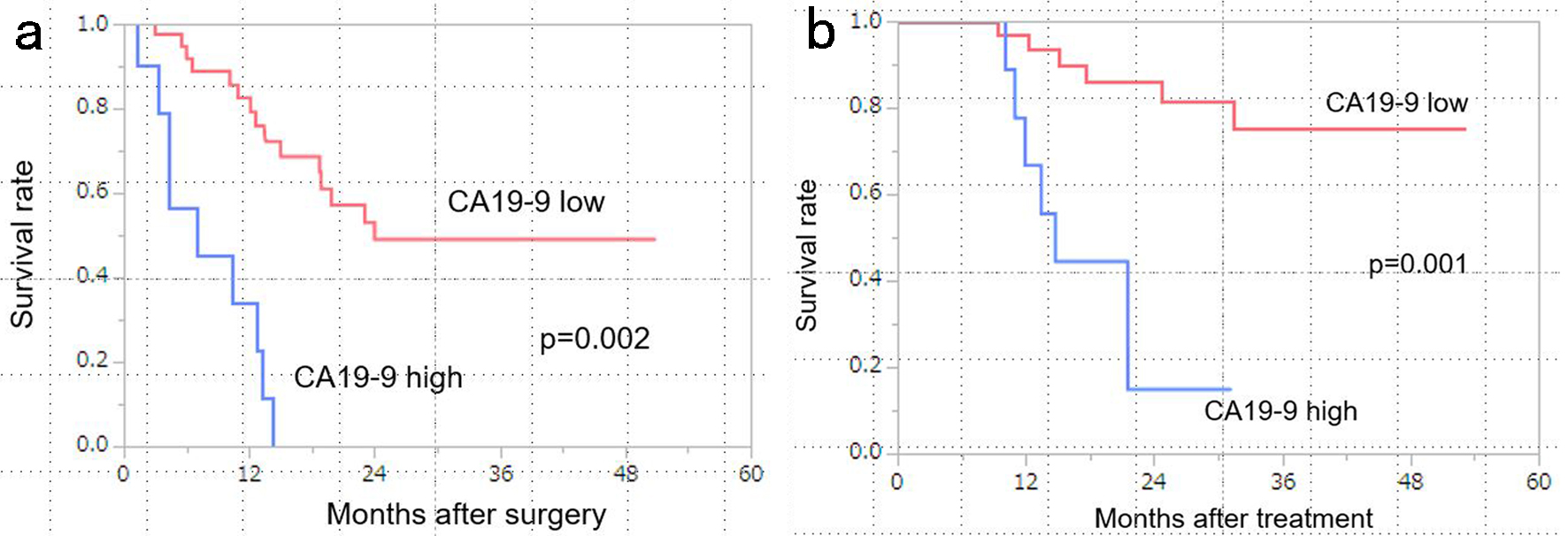
Figure 1. Kaplan-Meier curves stratified by CA19-9 levels (low vs. high). (a) Patients with low CA19-9 levels (< 100 U/mL) had significantly better disease-free survival (DFS) than those with high CA19-9 levels (median survival time (MST): low CA19-9, 24 months vs. high CA19-9, 7.0 months; P = 0.002). (b) Patients with low CA19-9 levels (< 100 U/mL) had significantly better overall survival (OS) than those with high CA19-9 levels (MST: low CA19-9, not reached vs. high CA19-9, 14.7 months; P = 0.001). CA19-9: carbohydrate antigen 19-9.