| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 000, Number 000, January 2025, pages 000-000
Angiogenesis Is Associated With Aggressive Biology That Counterbalances With Tumor Immunogenicity in Hepatocellular Carcinoma
Raj Vaghjiania, h, Rongrong Wua, b, h, Kaity H. Tunga, c, Takashi Ishikawab, Kazuaki Takabea, b, c, d, e, f, g, i
aDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
bDepartment of Breast Surgery and Oncology, Tokyo Medical University, Tokyo, Japan
cDepartment of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, The State University of New York, Buffalo, NY, USA
dDepartment of Surgery, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan
eDepartment of Gastroenterological Surgery, Yokohama City University School of Medicine, Yokohama, Japan
fDepartment of Breast Surgery, Fukushima Medical University, Fukushima, Japan
gDepartment of Immunology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
hThese authors contributed equally to this work.
iCorresponding Author: Kazuaki Takabe, Department of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14203, USA
Manuscript submitted December 9, 2024, accepted January 17, 2025, published online January 25, 2025
Short title: Angiogenesis and Immunogenicity in HCC
doi: https://doi.org/10.14740/wjon2009
| Abstract | ▴Top |
Background: Hepatocellular carcinoma (HCC) is an arterialized tumor; thus, anti-angiogenesis targeted therapy is in clinical practice. Herein, we hypothesized that HCC with high angiogenesis is biologically aggressive with worse survival.
Methods: Angiogenesis score (AS) was derived from the Molecular Signatures Database (MSigDB) Hallmark Angiogenesis Gene Set, and median was used to divide high versus low groups. Transcriptome of HCC patients of The Cancer Genome Atlas (TCGA, n = 386) and GSE76427 (n = 115) cohorts were analyzed.
Results: High AS correlated with angiogenesis-related gene expressions. Both microvascular and lymphatic endothelial cell infiltrations were higher in high angiogenesis HCC. Surprisingly, no survival difference was seen with varying levels of angiogenesis. High angiogenesis significantly enriched tumor aggravating signaling pathways: glycolysis, Notch, Hedgehog, KRAS, epithelial mesenchymal transition, and transforming growth factor-beta (TGF-β) in Gene Set Enrichment Analysis (GSEA), but also infiltrated less CD8+ T cells and T-helper 1 cells, and higher M1 macrophages and conventional dendritic cells (cDCs) with elevated cytolytic activity score in both cohorts. In agreement, immune response-related gene sets: inflammatory response, tumor necrosis factor-alpha (TNF-α) signaling, allograft rejection, interferon-alpha, and interferon-gamma were all enriched to high angiogenesis HCC. Programmed cell death protein 1 (PD1), programmed death ligand 1 (PD-L1), programmed death ligand 2 (PD-L2), and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) were higher in high angiogenesis HCC in TCGA, but not in GSE76427 cohort.
Conclusions: Angiogenesis quantified using transcriptome of HCC patients demonstrated that it is associated with aggressive biology but also with tumor immunogenicity and immune response that counterbalance and did not reflect in survival. Given high expression of immune checkpoint molecules, we cannot help but speculate that immunotherapy may be useful for high angiogenesis HCC patients.
Keywords: Hepatocellular carcinoma; Angiogenesis; Immunogenicity
| Introduction | ▴Top |
Despite the recent advances in hepatocellular carcinoma (HCC) treatment, mortality remains high making HCC the third leading cause of cancer-deaths worldwide [1]. In early-stage disease, resection remains the cornerstone of treatment, but for those with advanced or recurrent disease, therapeutic options become more limited. With the recent burgeoning of immunotherapy, the potential for a more efficacious treatment paradigm in HCC is promising. Yet the complex physiological role of the liver makes understanding the interplay between angiogenesis and the host immune response paramount to efficient patient selection and treatment application.
HCC is known to develop in a multinodular fashion within inflamed parenchyma and accounts for 90% of malignant tumors found in the liver [2]. It tends to display a relatively arterialized, angiogenic phenotype with the tumor relying on new blood vessel formation for establishment and persistence within the liver parenchyma [3, 4]. This mechanism has served as the basis for traditional anti-angiogenic therapy including systemic agents that target the vascular endothelial growth factor (VEGF) pathway and for direct action modalities such as transcatheter arterial chemoembolization (TACE). Further, the liver is a key actor in host immune response and acts as a vast repository of immune cells and producers of pro-inflammatory cytokines, molecules, and proteins. The close relationship between a tumor’s blood supply and the host’s immune response has been demonstrated across tumor types and understanding this relationship using a large human cohort is critical to designing the next phase of HCC treatment [5].
Recent advance in bioinformatic analyses allows us to elucidate the clinical relevance of gene expressions [6-8] or cell infiltration [9-12] in cancer using transcriptomics. Genes Set Variation Analysis (GSVA) has been used to investigate a host of biological pathways as a way to understand the cumulative expression of genes (gene sets) as they apply to host effector function [12-19]. Using this methodology, an angiogenesis pathway score has quantified this exceedingly intricate physiological function as it takes places within the distinctly aberrant setting of HCC tumor establishment in a large cohort of human patients. In this investigation, angiogenesis is hypothesized to be a key driver of HCC immunogenicity and immune checkpoint expression.
| Materials and Methods | ▴Top |
HCC cohort
The Cancer Genome Atlas (TCGA) clinical and RNA-sequencing data for 386 patients with HCC were downloaded via the cBioportal as we described previously [4, 6, 9, 19, 20]. An additional validation cohort of 115 HCC patients (GSE76427) with clinical and RNA-sequencing data was acquired from the National Center for Biotechnology (NCBI) Gene Expression Omnibus (GEO). IRB exemption was granted from the Institutional Review Board at Roswell Park Comprehensive Cancer Center given the publicly available and de-identified nature of the data sets.
Gene Set Enrichment Analysis (GSEA) and GSVA
The Molecular Signatures Database (MSigDB) hallmark annotated gene set for angiogenesis (HALLMARK_ANGIOGENESIS) was used to derive the angiogenesis score (AS) as previously described [21, 22]. GSEA was carried out utilizing software from a joint project of UC San Diego and the Broad Institute [23]. Patients were stratified into high versus low score cohorts using a median AS value. It is known that receiver operating characteristic (ROC) curve is commonly used as an optimal cutoff to maximize the difference below and above the cutoff; however, we chose median as the cutoff because our objective of this study was not to report that our AS as a prognostic biomarker, but rather to investigate the biological differences between HCC with high vs. low angiogenesis. Stratification of AS based on median value is simple and reproducible. Its use as cutoff is also independent of clinical outcome, avoiding potential biases in selecting a cutoff based on specific dataset being analyzed. Its non-dependent nature allows better comparability and generalizability between different cohorts. A false discovery rate (FDR) of < 0.25 was deemed significant [24].
Cellular infiltration analysis and cytolytic activity (CYT) score
A gene signature-based method, xCell [25], was used to estimate the infiltration patterns of 64 immune and stromal cell types [26-31]. The immune CYT score was defined as a quantitative measure based on transcript levels of granzyme A (GZMA) and perforin (PRF1) [32].
Statistical analysis
The Kaplan-Meier method with the log-rank test was used to compare overall survival (OS) and disease-free survival (DFS) between the high and low AS groups. P < 0.05 was considered significant and all analysis was carried out using R [33] and Bioconductor [34].
| Results | ▴Top |
Elevated AS is associated with increased expression of angiogenesis-related genes
To investigate if the AS can serve as a reasonable proxy for intra-tumoral vasculogenic activity, we first examined the gene expression of known angiogenesis-related proteins (Fig. 1). In both the TCGA and GSE76427 cohorts, those tumors with high AS also had increased expression of vascular endothelial growth factor (VEGFC). Additionally, high AS tumors also demonstrated elevated endothelial cell markers: platelet endothelial cell adhesion molecule (PECAM-1, CD31) and von Willebrand factor (VWF). Finally, higher levels of vascular stability genes including tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 1 and 2 (TIE1 and 2), angiopoietin 1 (ANGPT1), vascular endothelial cadherin (CD144, VE-cadherin), Claudin5 (CLDN5) and junction adhesion molecule 2 (JAM2) were found in tumors with high AS. The expression of these proteins was distinct from the genes which were used to derive the “HALLMARK_ANGIOGENESIS” annotated gene set, lending credence to the viability of the AS as a metric of intra-tumoral angiogenesis.
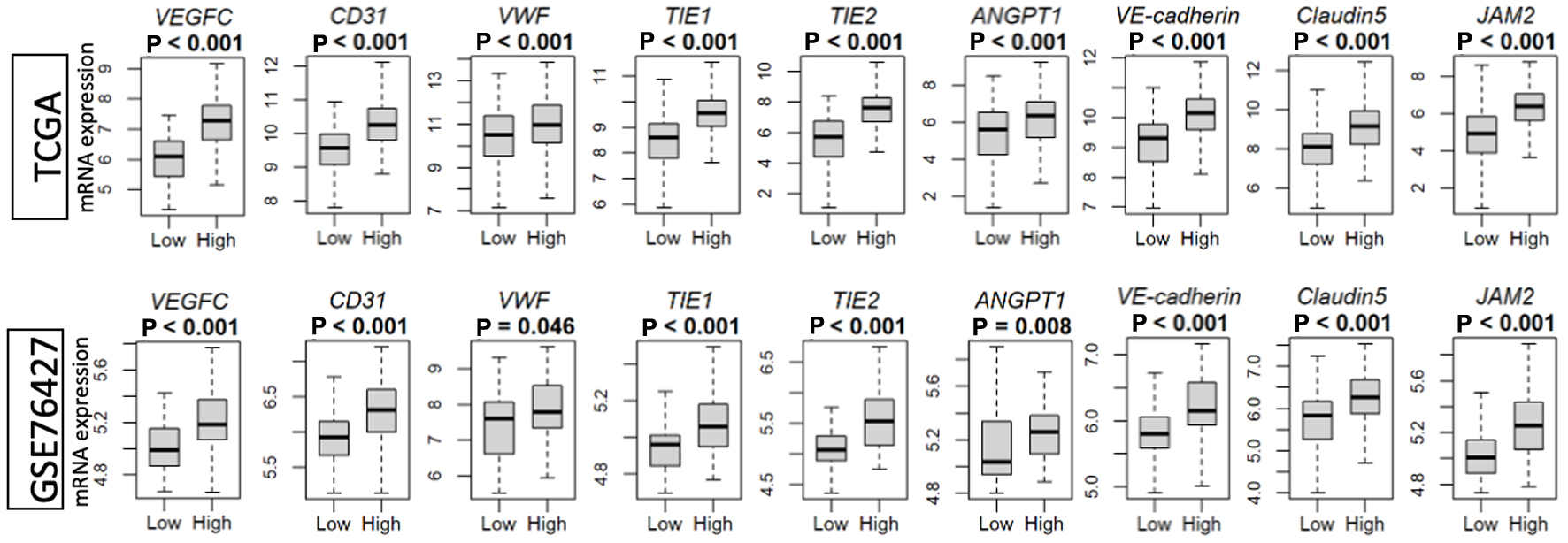 Click for large image | Figure 1. Intra-tumoral angiogenesis score correlates with the expression of VEGF (VEGFC)-, endothelial cell marker (CD31 and VWF)-, and vascular stability (TIE1 and 2, ANGPT1, VE-cadherin, CLDN5 and JAM2)-related genes in the TCGA and GSE76427 cohorts. High versus low, median angiogenesis pathway score. ANGPT1: angiopoietin 1; CD31/PECAM-1: platelet endothelial cell adhesion molecule; CLDN5: Claudin5; JAM2: junction adhesion molecule 2; TIE1 and 2: tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 1 and 2; VE-cadherin/CD144: vascular endothelial cadherin; VEGF: vascular endothelial growth factor; VWF: von Willebrand factor. |
Next, the structural foundation of angiogenesis was investigated. Using the gene set derived infiltration signatures from the xCell method, both microvascular endothelial cell (MEC) and lymphatic endothelial cell (LEC) expression levels were measured in both the TCGA and GSE76427 cohorts. MEC and LEC infiltration were found to be significantly increased in tumors which had high AS (Fig. 2).
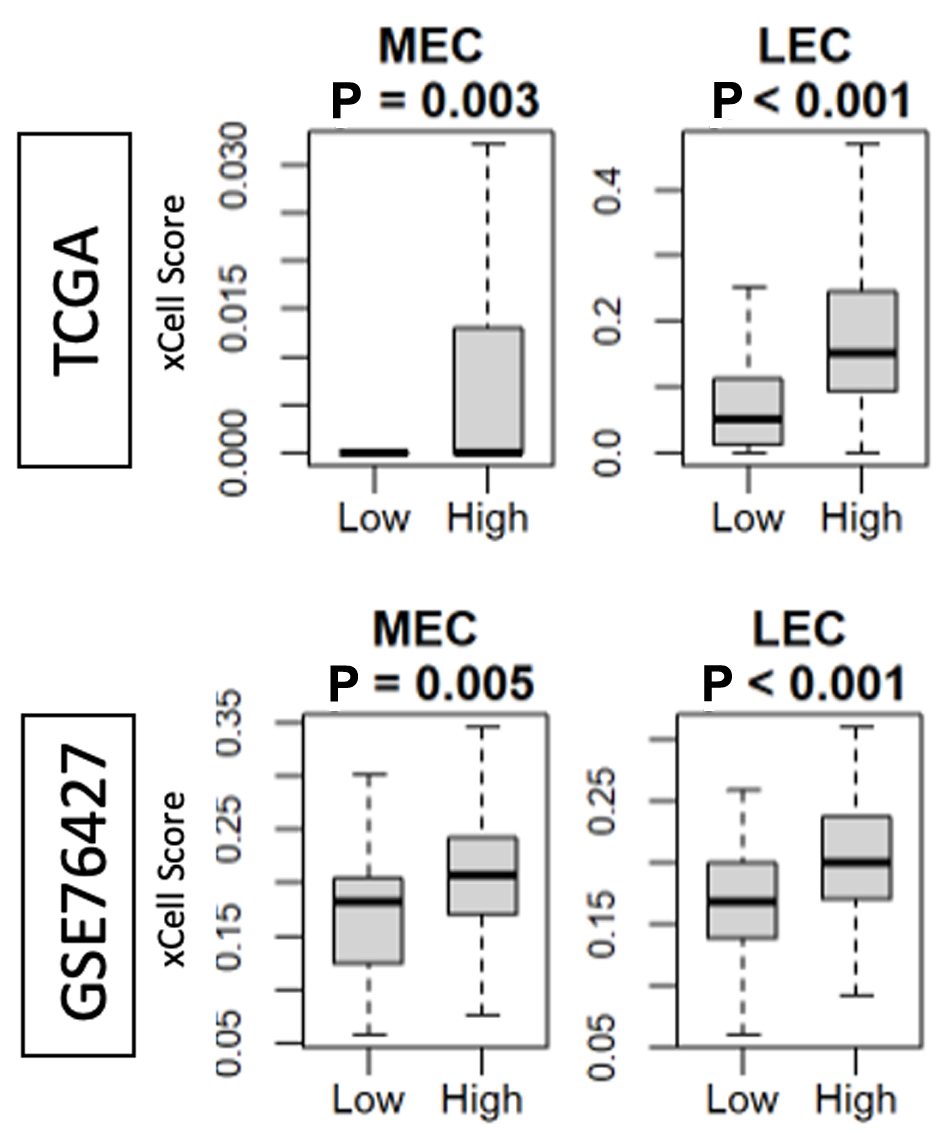 Click for large image | Figure 2. Intra-tumoral angiogenesis score correlates with the presence of MEC and LEC in the TCGA and GSE76427 cohorts. High versus low, median angiogenesis pathway score. MEC: microvascular endothelial cell; LEC: lymphatic endothelial cell. |
AS does not augment patient survival
In advanced, non-operable HCC, antiangiogenic therapy forms a cornerstone of therapeutic approach. Mechanistically, aberrant and uncontrolled angiogenesis contributes to tumor sustenance and thus it could stand to reason that those tumors with more angiogenesis may indeed have worsened outcomes. To investigate this, the survival outcomes of HCC patients were examined (Fig. 3). No differences were appreciated between patients who had tumors with high versus low AS in terms of DFS and OS. However, early in the course of HCC within 3 years of resection, there appears to be an interval during which patients whose tumors had a higher AS did demonstrate a survival benefit that eventually abrogated as time progressed.
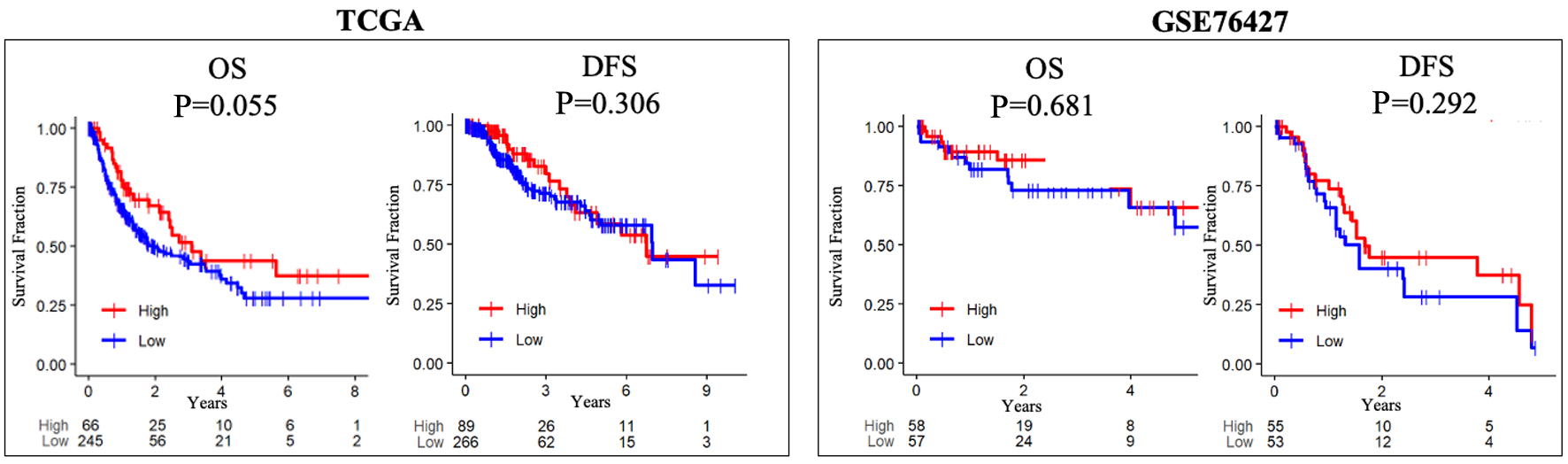 Click for large image | Figure 3. Intra-tumoral angiogenesis score does not correlate with OS or DFS in either the TCGA and GSE76427 cohorts. High versus low, median angiogenesis pathway score. DFS: disease-free survival; OS: overall survival. |
A high AS significantly enriches pro-tumor gene sets
To further investigate the mechanistic role of angiogenesis in HCC, GSEA was performed to examine cellular pathway in both the TCGA and GSE76427 cohorts (Fig. 4). Tumors with a high AS enriched glycolysis and epithelial mesenchymal transition (EMT) gene sets. Additionally high AS tumors also significantly enriched Notch, Hedgehog, KRAS, and transforming growth factor-beta (TGF-β) signaling gene sets.
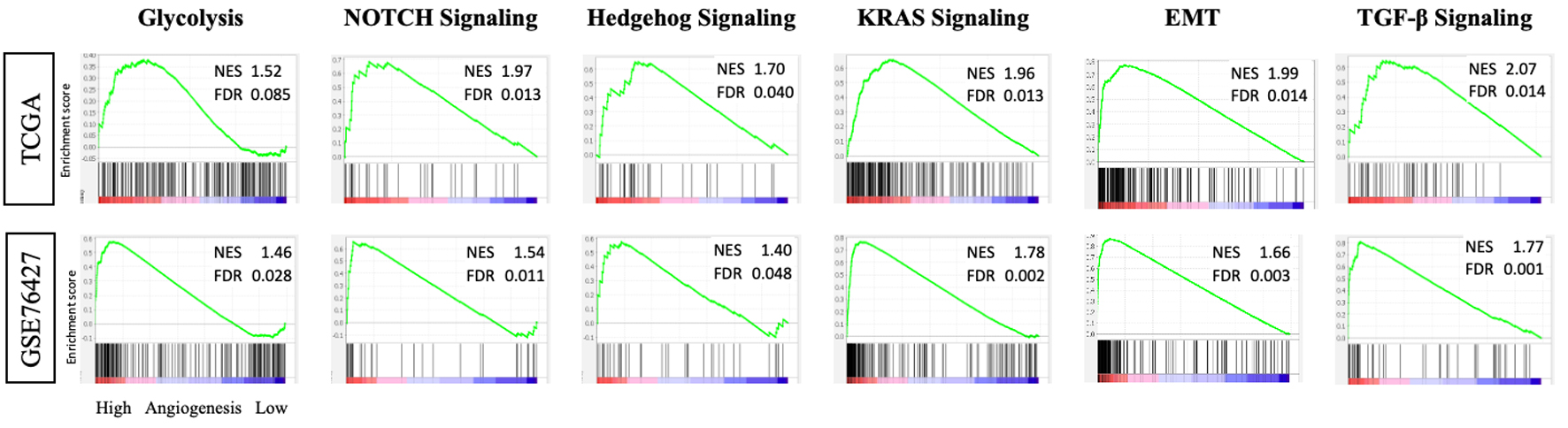 Click for large image | Figure 4. Tumors with high intra-tumoral angiogenesis score demonstrate enrichment of pro-tumor gene sets in the TCGA and GSE76427 cohorts. GSEA with NES and FDR for glycolysis, Notch signaling, Hedgehog signaling, KRAS signaling, EMT, and TGF-β signaling. EMT: epithelial mesenchymal transition; FDR: false discovery rate; GSEA: Gene Set Enrichment Analysis; NES: normalized enrichment score; TGF-β: transforming growth factor-beta. |
High angiogenesis HCC is associated with less lymphocytes and higher myeloid cells, and summative CYT score is significantly elevated
As tumor angiogenesis is not the sole drive of progression, we sought to examine one other major contributor to tumor persistence: the host immune response. To investigate the impact of AS on the tumor microenvironment (TME), the cellular infiltration patterns of tumors were examined using the xCell algorithm in both TCGA and GSE cohorts. Interestingly, the infiltration patterns were variable in terms of a pro- or anti-tumor environment (Fig. 5a). CD8+ lymphocytes and T-helper 1 (Th1) cells were more prominent in tumors with low AS. Additionally macrophages, specifically M1 type, and conventional dendritic cells (cDC) were prevalent in tumors with high AS. In order to elucidate the cumulative effect of this cellular infiltration pattern, we calculated the CYT score based on the quantitative measure of transcript levels of GZMA and PRF1. Tumors with high AS had a higher CYT score in both cohorts (Fig. 5b).
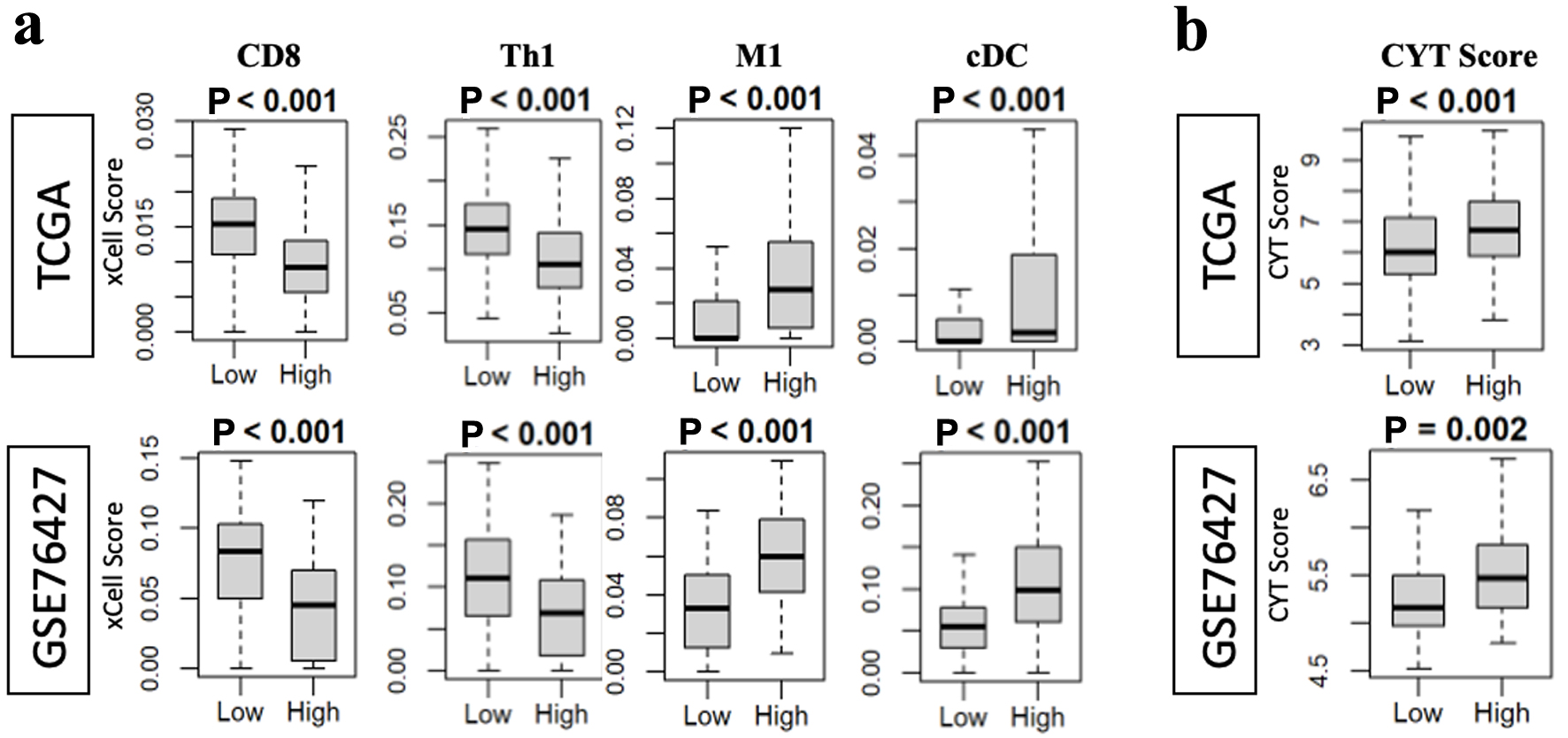 Click for large image | Figure 5. High angiogenesis score is associated with increased CYT in both the TCGA and GSE76427 cohorts, while pro-and anti-tumor infiltrating immune cells showed variable presence. (a) CD8 and Th1 infiltration was associated with low angiogenesis score while M1 macrophages and cDC infiltration was associated with high angiogenesis score. (b) CYT score was correlated with tumors which had high angiogenesis score. cDC: conventional dendritic cell; CYT: cytolytic activity. |
High angiogenesis tumors enrich immune response-related gene sets
Since the CYT score was elevated in tumors with high AS, then the adjunct cellular pathways associated with increased reactionary inflammation should also show a similar pattern. GSEA was applied to tumors with high AS and inflammatory response gene sets were examined (Fig. 6). Signaling pathways associated with tumor necrosis factor-alpha (TNF-α) and allograft rejection were enriched in both cohorts. Additionally, the interferon-alpha/gamma (INF-α/γ) and inflammatory response pathways also demonstrated significant enrichment in high AS tumors.
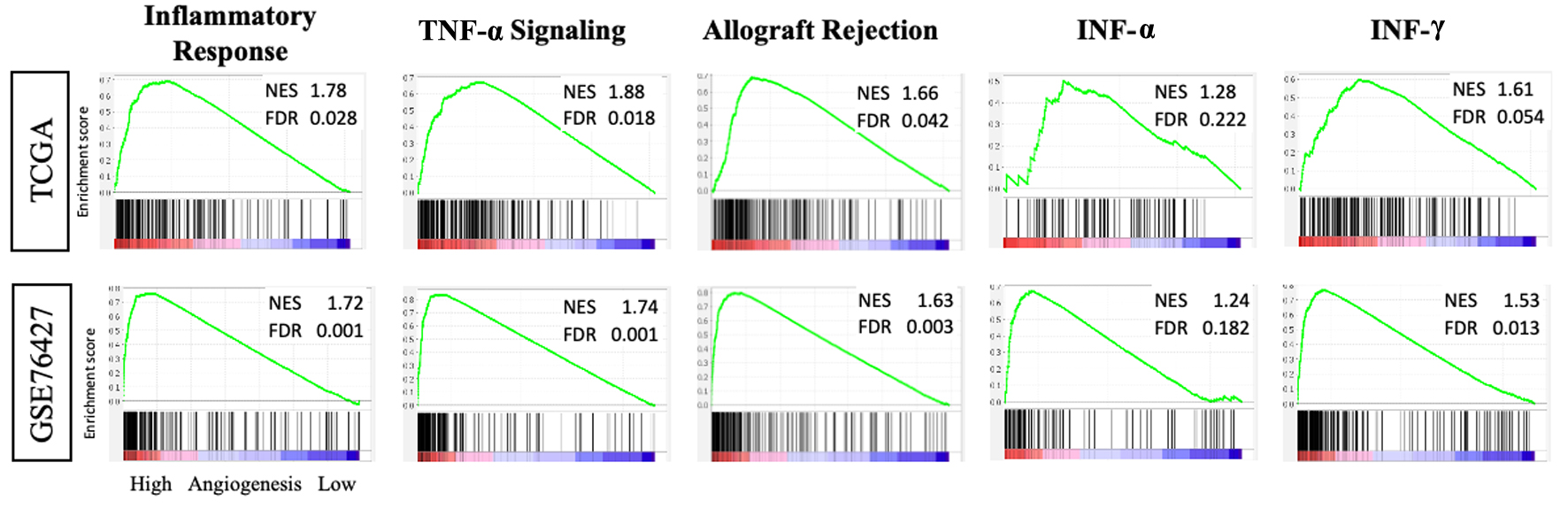 Click for large image | Figure 6. Tumors with high intra-tumoral angiogenesis score demonstrate enrichment of inflammatory response gene sets in the TCGA and GSE76427 cohorts. GSEA with NES and FDR for inflammatory response, TNF-α, allograft rejection, INF-α and γ. FDR: false discovery rate; GSEA: Gene Set Enrichment Analysis; INF-α and γ: interferon-alpha and gamma; NES: normalized enrichment score; TNF-α: tumor necrosis factor-alpha. |
High angiogenesis HCC shows increased immune checkpoint marker expression
Amongst patients whose tumors demonstrated high AS in the first 3 years, a transient survival benefit appears to develop and subsequently dissipate. This may indicate that there is a period of time during which intra-tumoral angiogenesis primes the host immune system for a transient anti-tumor response after which tumor escape leads to patient demise. To investigate this mechanism further, we sought to characterize the expression of checkpoint markers (Fig. 7). A high AS correlated with increased expression of programmed cell death protein 1 (PD1), programmed death ligand 1 (PD-L1), and programmed death ligand 2 (PD-L2) in the TCGA cohort. In both the TCGA and GSE cohorts, high tumor AS correlated with higher levels of cytotoxic T lymphocyte-associated protein 4 (CTLA-4).
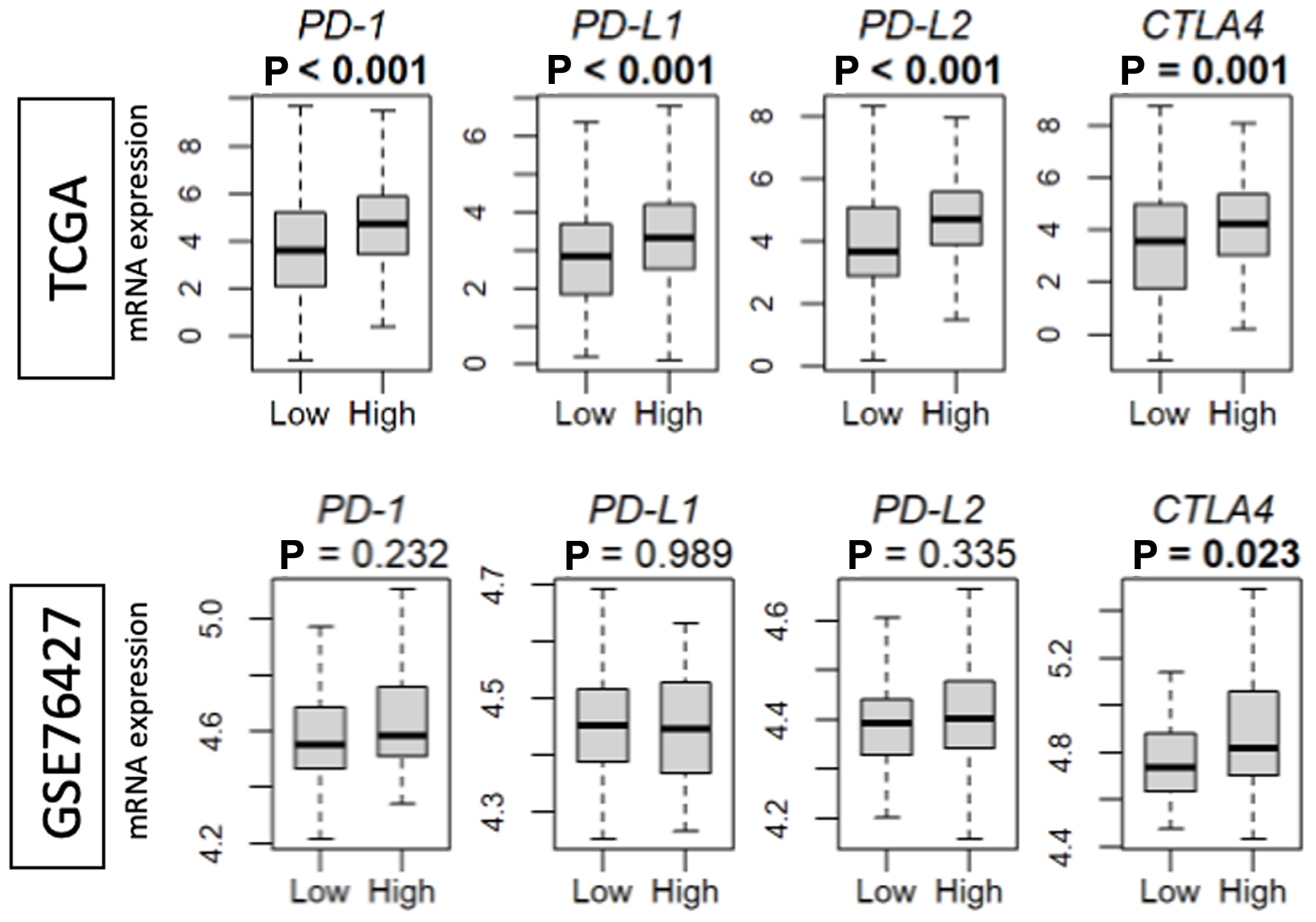 Click for large image | Figure 7. Intra-tumoral angiogenesis score correlates with checkpoint marker expression in the TCGA and GSE76427 cohorts. High versus low, median angiogenesis pathway score. PD1: programmed cell death protein 1; PD-L1 and 2: programmed death ligand 1 and 2; CTLA-4/CD152: cytotoxic T lymphocyte-associated protein 4. |
| Discussion | ▴Top |
The results of this study indicate that angiogenesis is a cornerstone in the immunogenic potential of HCC. The AS derived from the MSigDB hallmark annotated gene set was used to investigate two large patient cohorts. Tumors with high AS in both cohorts demonstrated higher expression of VEGF (VEGFC), endothelial cells markers (CD31, VWF), and vascular stability markers (TIE1 and 2, ANGPT1, VE-cadherin, CLDN5 and JAM2) leading credence to the score serving as a reasonable metric for tumor angiogenesis. In these cohorts, AS was not associated with clinical outcomes including DFS and OS. Additionally, tumors with high AS showed enrichment of classic pro-tumor gene sets including Notch, Hedgehog, KRAS, TGF-β, glycolysis and EMT. The TME within these patient cohorts were variable, showing both pro- and anti-tumor signatures; however, the aggregate effect as measured by CYT was elevated in tumors with a high AS. High AS tumors also showed enrichment of the pro-inflammatory gene sets IFN-α/γ, TNF-α, allograft rejection and inflammatory response. Finally, both cohorts showed that tumors with higher AS also had increased immune checkpoint marker expression including PD1, PD-L1, PD-L2, and CTLA-4.
The current paradigm for the treatment of HCC hinges on anatomic resectability [35]. However, up to 70% of patients worldwide may not be candidates for surgery or transplantation at diagnosis; thus, the treatment of most patients relies on a combination of local therapies (i.e., ablation, transarterial chemoembolization, transarterial radioembolization, etc.) and systemic therapy [36, 37]. Unfortunately, traditional chemotherapeutics have shown suboptimal efficacy for HCC. In the contrary, metronomical delivery of anti-angiogenic chemotherapy, such as capecitabine, demonstrated significant anti-tumor efficacy with a good safety profile and significant radiological tumor response in cases of HCC that failed sorafenib [38-42]. Thus, current management guidelines are based on targeted anti-angiogenic agents and, more recently, immunotherapeutics [43-45]. However, immunotherapy has associated complications; therefore, a metric to better select patients is needed to reduce those complications [46].
Although overall HCC tends to be a malignancy characterized by arterialization, this study identifies a subset of patients whose tumors may reside at the higher end of this spectrum, and in turn exhibit a more immunogenic signature [47]. Other investigations have shown similarly that HCC may be an immunologically heterogenous disease, with some tumors being at least theoretically more susceptible to checkpoint therapy [48]. Current studies indicate that immunotherapy may be effective in up to 30% of patients, but there is no distinct maker to identify immunotherapy candidacy [49]. Here, we suggest that the AS may provide a way to identify tumors whose transcriptomic signature and aggregate cellular infiltration pattern may lead to increased susceptibility to immune modulation. In-vivo investigation could readily be supported by the framework established by AS in this investigation. The results of this study further support the potential therapeutic impact of enhanced anti-tumor effects by combination strategy of tyrosine kinase inhibitors as antiangiogenic agents and immune checkpoint inhibitors. This dual synergistic effect has been recently reviewed, addressing the rationale of ongoing clinical trials [50].
There are some limitations to the interpretation of our results. This was a retrospective analysis performed on banked and cataloged tissue and transcriptomic data. As demonstrated by other investigations, the intra-tumoral and peri-tumoral tissue can be highly heterogeneous, and thus, tissue sampling error may play a role. The cohorts examined only had limited clinical annotation, and thus, extrapolation to highly selected patients is difficult. It has been reported that there is variability in HCC-related angiogenesis and microenvironment by underlying liver disease. It would be ideal to document the underlying liver disease in TCGA and GSE7642733 cohorts; however, we did not have access to such data at this time. Additionally, whether the single or multiple nodules of HCC have a clinical impact on this study is of interest. However, we do not have access to number of nodules of HCC on the cohorts we used, and we were unable to conduct such analyses. Finally, the AS is a derivative score that would require prospective validation before clinical application.
In conclusion, the angiogenesis score in HCC appears to be a reasonable metric for intra-tumoral angiogenesis. HCC, which has high AS, may be pro-immunogenic, and thus, this score may help stratify patients in which a more selective application of immunotherapy could be achieved.
Acknowledgments
None to declare.
Financial Disclosure
This research was supported by National Institutes of Health, USA (grant number R01CA160688, R37CA248018, R01CA250412, R01CA251545, and R01EB029596), as well as US Department of Defense BCRP (grant number W81XWH-19-1-0674 and W81XWH-19-1-0111) to K. Takabe. National Cancer Institute, cancer center support grant P30CA016056 to Roswell Park Comprehensive Cancer Center.
Conflict of Interest
The authors have no potential conflict of interest to disclose.
Informed Consent
Not applicable.
Author Contributions
Conceptualization: R. Vaghjiani, R. Wu, and K. Takabe. Data analyses: R. Vaghjiani, R. Wu, and K. Takabe. Writing - original draft preparation: R. Vaghjiani. Writing - review and editing: K. Tung, T. Ishikawa, and K. Takabe. Supervision: T. Ishikawa and K. Takabe. Funding acquisition: K. Takabe. All authors have read and agreed to the published version of the manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author, KT, upon reasonable request.
Abbreviations
ANGPT1: angiopoietin 1; CD31/PECAM-1: platelet endothelial cell adhesion molecule; cDC: conventional dendritic cell; CD144/VE-cadherin: vascular endothelial cadherin; CD152/CTLA-4: cytotoxic T lymphocyte-associated protein 4; CLDN5: Claudin5; CYT: cytolytic activity; DFS: disease-free survival; EMT: epithelial mesenchymal transition; FDR: false discovery rate; GSEA: Gene Set Enrichment Analysis; HCC: hepatocellular carcinoma; INF-α and γ: interferon-alpha and gamma; JAM2: junction adhesion molecule 2; LECs: lymphatic endothelial cells; MECs: microvascular endothelial cells; NES: normalized enrichment score; OS: overall survival; PD1: programmed cell death protein 1; PD-L1 and 2: programmed death ligand 1 and 2; TIE1 and 2: tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 1 and 2; TNF-α: tumor necrosis factor-alpha; VEGF: vascular endothelial growth factor; VWF: von Willebrand factor
| References | ▴Top |
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263.
doi pubmed - Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2362-2368.
doi pubmed - Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353-364.
doi pubmed - Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, Finn RS. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res. 2019;25(3):912-920.
doi pubmed - Hendry SA, Farnsworth RH, Solomon B, Achen MG, Stacker SA, Fox SB. The role of the tumor vasculature in the host immune response: implications for therapeutic strategies targeting the tumor microenvironment. Front Immunol. 2016;7:621.
doi pubmed - Ramos-Santillan V, Oshi M, Nelson E, Endo I, Takabe K. High Ki67 gene expression is associated with aggressive phenotype in hepatocellular carcinoma. World J Oncol. 2024;15(2):257-267.
doi pubmed - Satyananda V, Oshi M, Tokumaru Y, Maiti A, Hait N, Matsuyama R, Endo I, et al. Sphingosine 1-phosphate (S1P) produced by sphingosine kinase 1 (SphK1) and exported via ABCC1 is related to hepatocellular carcinoma (HCC) progression. Am J Cancer Res. 2021;11(9):4394-4407.
pubmed - Takahashi K, Yan L, An N, Chida K, Tian W, Oshi M, Takabe K. RAD51 high-expressed hepatocellular carcinomas are associated with high cell proliferation. J Surg Res. 2024;302:250-258.
doi pubmed - Cherkassky L, Oshi M, Abdelfatah E, Wu R, Takabe Y, Yan L, Endo I, et al. An immune-inflamed tumor microenvironment as defined by CD8 score is associated with favorable oncologic outcomes in hepatocellular carcinoma independent of measures of tumor mutational burden. Am J Cancer Res. 2022;12(7):3099-3110.
pubmed - Mukhopadhyay S, Tokumaru Y, Oshi M, Endo I, Yoshida K, Takabe K. Low adipocyte hepatocellular carcinoma is associated with aggressive cancer biology and with worse survival. Am J Cancer Res. 2022;12(8):4028-4039.
pubmed - Oshi M, Wu R, Khoury T, Gandhi S, Yan L, Yamada A, Ishikawa T, et al. Infiltration of common myeloid progenitor (CMP) cells is associated with less aggressive tumor biology, lower risk of brain metastasis, better response to immunotherapy, and higher patient survival in breast cancer. Ann Surg. 2024;280(4):557-569.
doi pubmed - Wu R, Oshi M, Asaoka M, Yan L, Benesch MGK, Khoury T, Nagahashi M, et al. Intratumoral tumor infiltrating lymphocytes (TILs) are associated with cell proliferation and better survival but not always with chemotherapy response in breast cancer. Ann Surg. 2023;278(4):587-597.
doi pubmed - Oshi M, Sarkar J, Tokumaru Y, Yan L, Kosaka T, Akiyama H, Nagahashi M, et al. Higher intra-tumoral expression of pro-coagulation genes is a predictor of angiogenesis, epithelial mesenchymal transition and worse patient survival in gastric cancer. Am J Cancer Res. 2022;12(8):4001-4014.
pubmed - Oshi M, Ziazadeh D, Wu R, Chida K, Yamada A, Yamamoto S, Narui K, et al. GALNT1 expression is associated with angiogenesis and is a prognostic biomarker for breast cancer in adolescents and young adults (AYA). Cancers (Basel). 2023;15(13):3489.
doi pubmed - Oshi M, Chida K, Roy AM, Mann GK, An N, Yan L, Endo I, et al. Higher inflammatory response in hepatocellular carcinoma is associated with immune cell infiltration and a better outcome. Hepatol Int. 2024;18(4):1299-1309.
doi pubmed - Patel A, Oshi M, Yan L, Matsuyama R, Endo I, Takabe K. The unfolded protein response is associated with cancer proliferation and worse survival in hepatocellular carcinoma. Cancers (Basel). 2021;13(17):4443.
doi pubmed - Oshi M, Kim TH, Tokumaru Y, Yan L, Matsuyama R, Endo I, Cherkassky L, et al. Enhanced DNA repair pathway is associated with cell proliferation and worse survival in hepatocellular carcinoma (HCC). Cancers (Basel). 2021;13(2):323.
doi pubmed - Takahashi H, Kawaguchi T, Yan L, Peng X, Qi Q, Morris LGT, Chan TA, et al. Immune cytolytic activity for comprehensive understanding of immune landscape in hepatocellular carcinoma. Cancers (Basel). 2020;12(5):1221.
doi pubmed - Chida K, Oshi M, Roy AM, Yachi T, Nara M, Yamada K, Matsuura O, et al. E2F target score is associated with cell proliferation and survival of patients with hepatocellular carcinoma. Surgery. 2023;174(2):307-314.
doi pubmed - Sarkar J, Oshi M, Satyananda V, Chida K, Yan L, Maiti A, Hait N, et al. Spinster homologue 2 expression correlates with improved patient survival in hepatocellular carcinoma despite association with lymph-angiogenesis. World J Oncol. 2024;15(2):181-191.
doi pubmed - Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1(6):417-425.
doi pubmed - Oshi M, Newman S, Tokumaru Y, Yan L, Matsuyama R, Endo I, Nagahashi M, et al. Intra-tumoral angiogenesis is associated with inflammation, immune reaction and metastatic recurrence in breast cancer. Int J Mol Sci. 2020;21(18):6708.
doi pubmed - http://www.gsea-msigdb.org.
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545-15550.
doi pubmed - https://xcell.ucsf.edu/.
- Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017;18(1):220.
doi pubmed - Oshi M, Tokumaru Y, Asaoka M, Yan L, Satyananda V, Matsuyama R, Matsuhashi N, et al. M1 Macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci Rep. 2020;10(1):16554.
doi pubmed - Oshi M, Asaoka M, Tokumaru Y, Angarita FA, Yan L, Matsuyama R, Zsiros E, et al. Abundance of regulatory T cell (Treg) as a predictive biomarker for neoadjuvant chemotherapy in triple-negative breast cancer. Cancers (Basel). 2020;12(10):3038.
doi pubmed - Oshi M, Newman S, Tokumaru Y, Yan L, Matsuyama R, Kalinski P, Endo I, et al. Plasmacytoid dendritic cell (pDC) infiltration correlate with tumor infiltrating lymphocytes, cancer immunity, and better survival in triple negative breast cancer (TNBC) more strongly than conventional dendritic cell (cDC). Cancers (Basel). 2020;12(11):3342.
doi pubmed - Oshi M, Huyser MR, Le L, Tokumaru Y, Yan L, Matsuyama R, Endo I, et al. Abundance of microvascular endothelial cells is associated with response to chemotherapy and prognosis in colorectal cancer. Cancers (Basel). 2021;13(6):1477.
doi pubmed - Chouliaras K, Oshi M, Asaoka M, Tokumaru Y, Khoury T, Endo I, Ishikawa T, et al. Increased intratumor heterogeneity, angiogenesis and epithelial to mesenchymal transition pathways in metaplastic breast cancer. Am J Cancer Res. 2021;11(9):4408-4420.
pubmed - Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1-2):48-61.
doi pubmed - https://www.r-project.org/.
- https://bioconductor.org/.
- Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, Garcia-Criado A, Kelley RK, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76(3):681-693.
doi pubmed - Sotiropoulos GC, Lang H, Frilling A, Molmenti EP, Paul A, Nadalin S, Radtke A, et al. Resectability of hepatocellular carcinoma: evaluation of 333 consecutive cases at a single hepatobiliary specialty center and systematic review of the literature. Hepatogastroenterology. 2006;53(69):322-329.
pubmed - Lau WY. Management of hepatocellular carcinoma. J R Coll Surg Edinb. 2002;47(1):389-399.
pubmed - Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105(8):1045-1047.
doi pubmed - Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4(6):423-436.
doi pubmed - Marinelli S, Granito A, Piscaglia F, Renzulli M, Stagni A, Bolondi L. Metronomic capecitabine in patients with hepatocellular carcinoma unresponsive to or ineligible for sorafenib treatment: report of two cases. Hepat Mon. 2013;13(9):e11721.
doi pubmed - Granito A, Marinelli S, Terzi E, Piscaglia F, Renzulli M, Venerandi L, Benevento F, et al. Metronomic capecitabine as second-line treatment in hepatocellular carcinoma after sorafenib failure. Dig Liver Dis. 2015;47(6):518-522.
doi pubmed - Trevisani F, Brandi G, Garuti F, Barbera MA, Tortora R, Casadei Gardini A, Granito A, et al. Metronomic capecitabine as second-line treatment for hepatocellular carcinoma after sorafenib discontinuation. J Cancer Res Clin Oncol. 2018;144(2):403-414.
doi pubmed - Le Grazie M, Biagini MR, Tarocchi M, Polvani S, Galli A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J Hepatol. 2017;9(21):907-920.
doi pubmed - Sangro B, Sarobe P, Hervas-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(8):525-543.
doi pubmed - Liu Z, Liu X, Liang J, Liu Y, Hou X, Zhang M, Li Y, et al. Immunotherapy for Hepatocellular Carcinoma: Current Status and Future Prospects. Front Immunol. 2021;12:765101.
doi pubmed - Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86-104.
doi pubmed - Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S, Sun L, et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol. 2023;78(4):770-782.
doi pubmed - Mattos AZ, Debes JD, Boonstra A, Vogel A, Mattos AA. Immune aspects of hepatocellular carcinoma: From immune markers for early detection to immunotherapy. World J Gastrointest Oncol. 2021;13(9):1132-1143.
doi pubmed - Foerster F, Gairing SJ, Ilyas SI, Galle PR. Emerging immunotherapy for HCC: A guide for hepatologists. Hepatology. 2022;75(6):1604-1626.
doi pubmed - Stefanini B, Ielasi L, Chen R, Abbati C, Tonnini M, Tovoli F, Granito A. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert Rev Anticancer Ther. 2023;23(3):279-291.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.