| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 000, Number 000, April 2025, pages 000-000
Nomograms for Predicting Overall Survival and Cancer-Specific Survival of Small Cell Carcinoma of Ovary Patients: A Retrospective Cohort Study
Chun Mei Yana, d, e, Ya Rong Chenb, d, Hong Fang Lic, Ri Cheng Lia
aDepartment of Gynaecology and Obstetrics, Hospital of Lanzhou Jiaotong University, Lanzhou, Gansu, China
bDepartment of Infectious Disease Prevention and Control, Chengdu Center for Disease Control and Prevention, Chengdu, Sichuan, China
cDepartment of Gynaecology and Obstetrics, The First People’s Hospital of Lanzhou, Lanzhou, Gansu, China
dThese authors contributed equally to this work and should be considered co-first authors.
eCorresponding Author: Chun Mei Yan, Department of Gynaecology and Obstetrics, Hospital of Lanzhou Jiaotong University, Lanzhou, Gansu, China
Manuscript submitted February 6, 2025, accepted April 3, 2025, published online April 22, 2025
Short title: Prognostic Nomograms for SCCO Patients
doi: https://doi.org/10.14740/wjon2543
| Abstract | ▴Top |
Background: This study aimed to develop functional nomograms to predict overall survival (OS) and cancer-specific survival (CSS) of small cell carcinoma of ovary (SCCO).
Methods: SSCO case data were recruited retrospectively from the Surveillance, Epidemiology, and End Results (SEER) database. Nomograms were constructed to predict the probabilities of OS and CSS in SCCO patients based on independent predictors. The predictive performance of nomogram was evaluated with the concordance index (C-index), area under the curve (AUC), calibration curves, and decision curve analysis (DCA).
Results: The independent risk factors affecting the prognosis of SCCO patients were older age, lower income, surgery, chemotherapy, radiation, advanced International Federation of Gynecology and Obstetrics (FIGO) stage, and number of primary tumors. The C-index for the OS nomogram was 0.78 (95% confidence interval (CI): 0.75 - 0.82), and AUCs for 1-, 3-, and 5-year OS were 0.861, 0.807, and 0.821, respectively. The C-index for the CSS nomogram was 0.79 (95% CI: 0.76 - 0.83), and AUCs for 1-, 3-, and 5-year OS were 0.873, 0.841, and 0.864, respectively. The calibration curves indicated reasonable agreement between the observed and predicted probabilities of the OS and CSS nomograms, which indicated a good degree of confidence. According to the C-index, ROC, and DCA, the prognostic nomograms of OS and CSS showed better prediction accuracy and clinical application value for SCCO than the FIGO staging system.
Conclusions: We constructed original nomograms that provided useful prediction of OS and CSS for patients with SCCO. These models could facilitate the postoperative personalized assessment and the identification of treatment strategy.
Keywords: Small cell carcinoma of ovary; SEER; Overall survival; Cancer-specific survival; Nomogram
| Introduction | ▴Top |
Small cell carcinoma of ovary (SCCO) is an extremely rare and highly aggressive malignant tumor, accounting for less than 1% of ovarian cancers [1]. SCCO can be divided into two subtypes: SCCO hypercalcemic type (SCCOHT) and SCCO pulmonary type (SCCOPT) [1]. The former is more commonly observed in young premenopausal women and is frequently associated with hypercalcemia [2]. The latter is even rarer and is primarily observed in postmenopausal women [3]. The prognosis is typically unfavorable [4], with less than a 40% cure rate even in the early stages of diseases [1]. There is no international consensus on medical therapy and surveillance, although various treatment approaches have been proposed [5].
Given the dearth of clinical data on this rare malignancy, the diagnosis, genetic counselling, epidemiology, and therapeutic strategies for SCCO remain controversial and warrant further investigation [6-8]. To date, fewer than 500 cases of SCCOHT have been reported in the literature [6], and most reports in the literature are based on single cases or clinicopathological analysis [9-11], and its rarity creates challenges in the identification and management of affected patients. Hence, it is crucial to identify the clinical features that are associated with a poorer outcome in SCCO. However, there is no consensus on the understanding of prognostic factors for SCCO and there is a lack of evaluated prognostic models.
The International Federation of Gynecology and Obstetrics (FIGO) staging system is a commonly used staging system for SCCO: the higher the stage, the lower the rate of survival. However, the FIGO staging system only takes into account the anatomical characteristics of the tumor and does not account for other factors of prognostic values regarding histological grading, age, ethnicity, and treatment. Thus, there are limitations in predicting the prognosis of SCCO by FIGO staging system alone.
As a simple and reliable statistical visual tool, the nomogram has been widely used in recent years to predict the prognosis and survival of some cancers [12-14]. Given the rarity of SCCO as well as its extremely poor prognosis, a large population-based study is essential [1-4]. The Surveillance, Epidemiology, and End Results (SEER) program, a large population-based public dataset, collects data on cancer incidence, treatment, and survival for approximately 30% of the US population, and benefits from extensive quality review [15]. In this study, all SCCO cases with clinicopathological and survival information were collected from 17 registries for the period 2000 - 2020 of the SEER program to establish intuitive and comprehensive nomograms for predicting survival in SCCO patients.
| Materials and Methods | ▴Top |
Data source and study population
We used data from the SEER database of 17 registries for the period 2000 - 2020. The SEER database collects data on cancer incidence, treatment, and survival for approximately 30% of the US population, and benefits from extensive quality review [15]. Ovarian cancer in the SEER database was identified via the site-specific International Classification of Oncological Diseases 3 (ICD-O-3) code C56.9 from 2000 to 2020. The diagnosis of SCCO was determined using the ICD-O-3 codes 8041-8045/3. Exclusion criteria were: 1) patients diagnosed only through autopsy or death certificate (n = 2); 2) unknown survival period or cause of death (n = 5); 3) the tumor was not primary (n = 28). A total of 236 SCCO patients were included in this study.
The study was approved by the Medical Ethics Committee of the First People’s Hospital of Lanzhou City (IRB no: 2024A-20). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. This study utilized publicly available data from the SEER database, and therefore informed consent was not required.
Definition of variables
Demographic information, clinical characteristics, and follow-up of survival of patients were extracted from the SEER database, including patient ID, age at diagnosis, marital status, median household income, race, year of diagnosis, tumor size, laterality, grade, FIGO stage, serum carbohydrate antigen 125 (CA125), lymph node status (LNS), sequence number, treatment (surgery status, chemotherapy, and radiotherapy), survival months, vital status, and cause-specific death. The outcome of the current study was overall survival (OS) and cancer-specific survival (CSS). OS was defined as the time interval between diagnosis and death from any cause. CSS was defined as the duration between diagnosis and death caused by SCCO.
Appropriate cutoff values for the variable age at diagnosis, year of diagnosis, and tumor size were assessed using the X-tile software. The variable age at diagnosis was then categorized into three groups: ≤ 45, 46 - 72, and ≥ 73 years. The variable year of diagnosis was divided into three groups: 2000 - 2002, 2003 - 2015, and 2016 - 2020, while tumor size was classified into ≤ 92, 93 - 141, and ≥ 142 mm groups (Fig. 1). Marital status was classified into four categories: single, married, divorced, separated, or widowed (DSW), and unknown. Median household income was classified into three categories: < $55,000, $55,000 - $69,999, and ≥ $70,000. Race was classified into six categories: non-Hispanic Black, non-Hispanic White, non-Hispanic Asian/Pacific Islander, non-Hispanic American Indian/Alaska Native, Hispanic, and non-Hispanic unknown race. Clinical characteristics were as follows: grade (I/II, III/IV, or unknown), FIGO stage (I, II, III, IV, or unknown), CA125 (negative, positive, or unknown), LNS (negative, positive, or unknown), and the number of primary tumors (one primary only versus first of two or more primaries). In alignment with SEER’s data structure, treatment variables were categorized as surgery status (yes vs. no), chemotherapy (yes vs. no/unknown), and radiotherapy (yes vs. no/unknown).
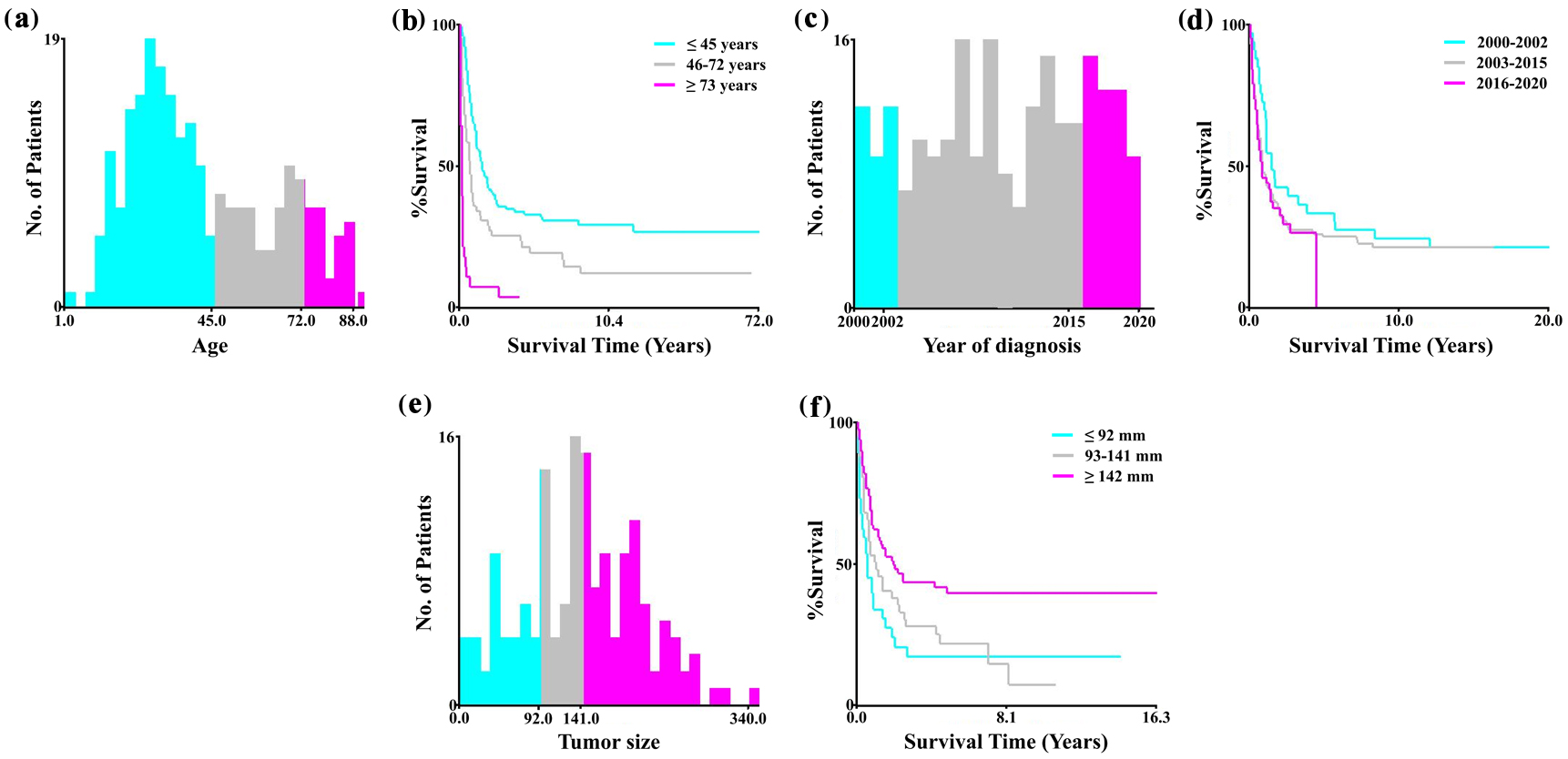 Click for large image | Figure 1. Optimal cutoff values for age, year of diagnosis, and tumor size using X-tile software analysis. (a, b) The optimal cutoff values of the variable age were 45 and 72 years. (c, d) The optimal cutoff values of the variable year of diagnosis were 2002 and 2015. (e, f) The optimal cutoff values of tumor size were 92 and 141 mm. |
Statistical analysis
Categorical data were shown as frequencies and percentages. A univariate Cox proportional hazards model was applied to explore the relationship between various demographic and clinical characteristics and the survival of patients. To identify independent predictors of OS and CSS, we further performed multivariate Cox regression analyses. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. The independent risk factors, identified by the multivariate analysis, were selected to construct nomograms for the prediction of the likelihood of OS and CSS.
We used the bootstrap validation method to estimate the discrimination capacity of the nomogram, which is presented by the concordance index (C-index) [16]. The larger the C-index, the more accurate the prognostic prediction was.
The calibration of nomograms for 1-, 3-, and 5-year OS and CSS were assessed by comparing predicted survival with observed survival. Receiver operating characteristic (ROC) curves were used to evaluate the predicting ability of OS and CSS nomograms by measuring the area under the ROC curve (AUC). Evaluation of the predictive power of the nomogram over time was conducted by applying ROC curves for 1-, 3-, and 5-year survival probability. The closer the AUC is to 1, the more accurate it is. To further evaluate the potential clinical benefit, the decision curve analysis (DCA) was used to assess the clinical decision utility and net benefit of the OS and CSS nomograms [17].
The data were analyzed and graphed using SPSS version 26.0 and R software version 4.4.0. We downloaded the following R packages to build nomograms and calculate C-index, AUC, plot calibration, and DCA curve: “survival”, “rms”, “pROC”, “timeROC”, “survcomp”, and “ggDCA”. All statistical analyses were set as two-sided, with P values < 0.05 set as the significance level.
| Results | ▴Top |
Patient characteristics
A total of 236 patients with SCCO in the SEER database were included in the study (Table 1). The mean follow-up period was 34 ± 54 months. Among them, 173 patients died, and 166 died because of cancer-specific causes. The majority of patients were non-Hispanic White people (63.98%), below the age of 46 (61.44%), single (44.92%), had a median income of at least $70,000 (47.88%), and had FIGO III (33.05%). The SCCO laterality was right-sided and left-sided in 96 (40.68%) and 84 (35.59%), respectively. Most patients were treated surgically (77.97%), with chemotherapy (76.69%), and without radiotherapy (89.41%). Baseline demographics and clinical features of the patients are shown in Table 1.
 Click to view | Table 1. Characteristics of Small Cell Carcinoma of Ovary Patients |
Risk factor analysis
Univariate Cox regression analysis showed that 10 variables, including age at diagnosis, marital status, median household income, laterality, tumor size, surgery status, chemotherapy status, radiation therapy, FIGO stage, and LNS were prognostic risk factors for both OS and CSS in patients with SCCO (Table 2). Additionally, the number of primary tumors was a prognostic factor for CSS (Table 2).
 Click to view | Table 2. Univariable Cox Regression for Analyzing the Associated Factors for Small Cell Carcinoma of Ovary |
Multivariate Cox regression analysis results indicated that, in general, older age (≥ 73 vs. ≤ 45, HR = 3.30), higher income ($55,000 - 69,999/≥ $70,000 vs. < $55,000, HR = 0.46/0.65), surgery status (surgery vs. no surgery, HR = 0.43), chemotherapy status (chemotherapy vs. no chemotherapy/unknown, HR = 0.33), and FIGO stage (II/III/IV vs. I, HR = 2.33/4.39/3.95) were determined as independent predictors associated with OS of SCCO (Table 3). Meanwhile older age (≥ 73 vs. ≤ 45, HR = 3.28), higher income ($55,000 - 69,999 vs. < $55,000, HR = 0.52), surgery status (surgery vs. no surgery, HR = 0.44), chemotherapy status (chemotherapy vs. no chemotherapy/unknown, HR = 0.35), radiation therapy (radiation vs. no radiation/unknown, HR = 0.49), FIGO stage (II/III/IV vs. I, HR = 2.44/4.03/4.54), and number of primary tumors (one primary only vs. first of two or more primaries, HR = 3.79) were determined as independent predictors associated with CSS of SCCO (Table 3).
 Click to view | Table 3. Multivariable Cox Regression for Analyzing the Associated Factors for Small Cell Carcinoma of Ovary |
Nomograms for prediction of OS and CSS
Subsequently, we constructed nomograms for 1-, 3-, and 5-year OS and CSS that incorporated all significant prognostic factors identified through multivariate analysis (Fig. 2). The nomograms showed that age, median household income, radiation therapy, surgery status, chemotherapy status, FIGO stage, and number of primary tumors were significant predictors of survival in patients with SCCO. Furthermore, we constructed nomograms of the predicted outcomes based exclusively on the FIGO staging system (Supplementary Material 1, wjon.elmerpub.com).
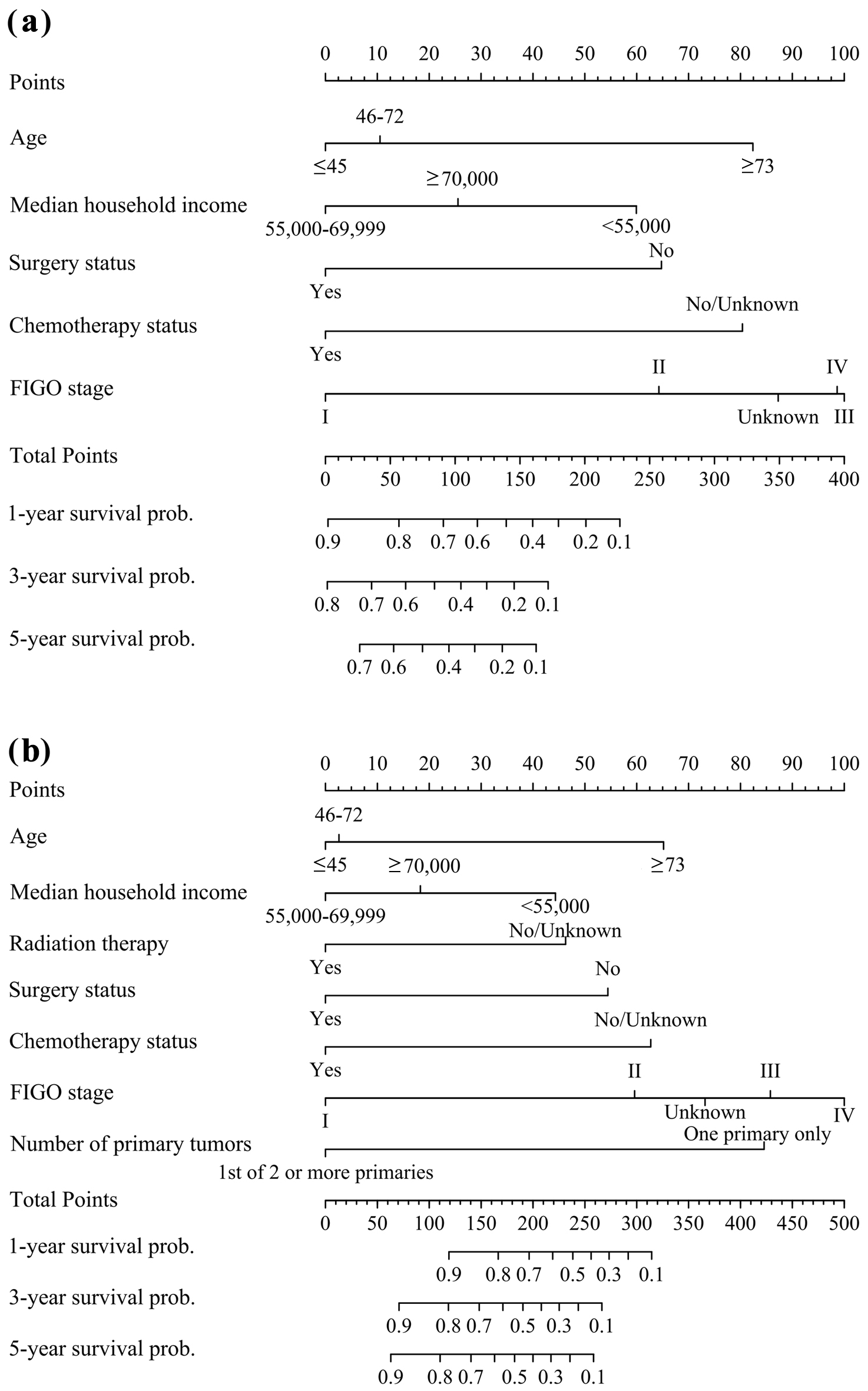 Click for large image | Figure 2. Nomograms for predicting 1-, 3-, and 5-year survival of SCCO patients: (a) overall survival; (b) cancer-specific survival. SCCO: small cell carcinoma of ovary; FIGO: Federation International of Gynecology and Obstetrics. |
Performance of nomograms
The C-indexes for the nomograms of OS and CSS were 0.78 (95% CI: 0.75 - 0.82) and 0.79 (95% CI: 0.76 - 0.83), respectively, both of which were greater than the nomograms based exclusively on the FIGO staging system (OS: 0.75 (95% CI: 0.70 - 0.80); CSS: 0.75 (95% CI: 0.70 - 0.80)).
To ensure that the nomogram forecast models had advantageous efficacy in predicting OS and CSS in SCCO patients, ROC analyses were performed. The 1-, 3-, and 5-year ROC curves showed that the nomogram model had significant value in predicting OS and CSS and outperformed the FIGO stages (Fig. 3). The calibration curves indicated reasonable agreement between the observed and predicted probabilities of the OS and CSS nomograms, which indicated a good degree of confidence (Fig. 4). DCA is a new method for evaluating alternative prognostic instruments that is superior to AUC. The DCA indicated that the predictive nomogram had high net benefits, implying that it has good clinical implementation in predicting the OS and CSS (Fig. 5). In addition, the DCA of the former appears to have better clinical application worth than the FIGO-based nomogram (Fig. 5).
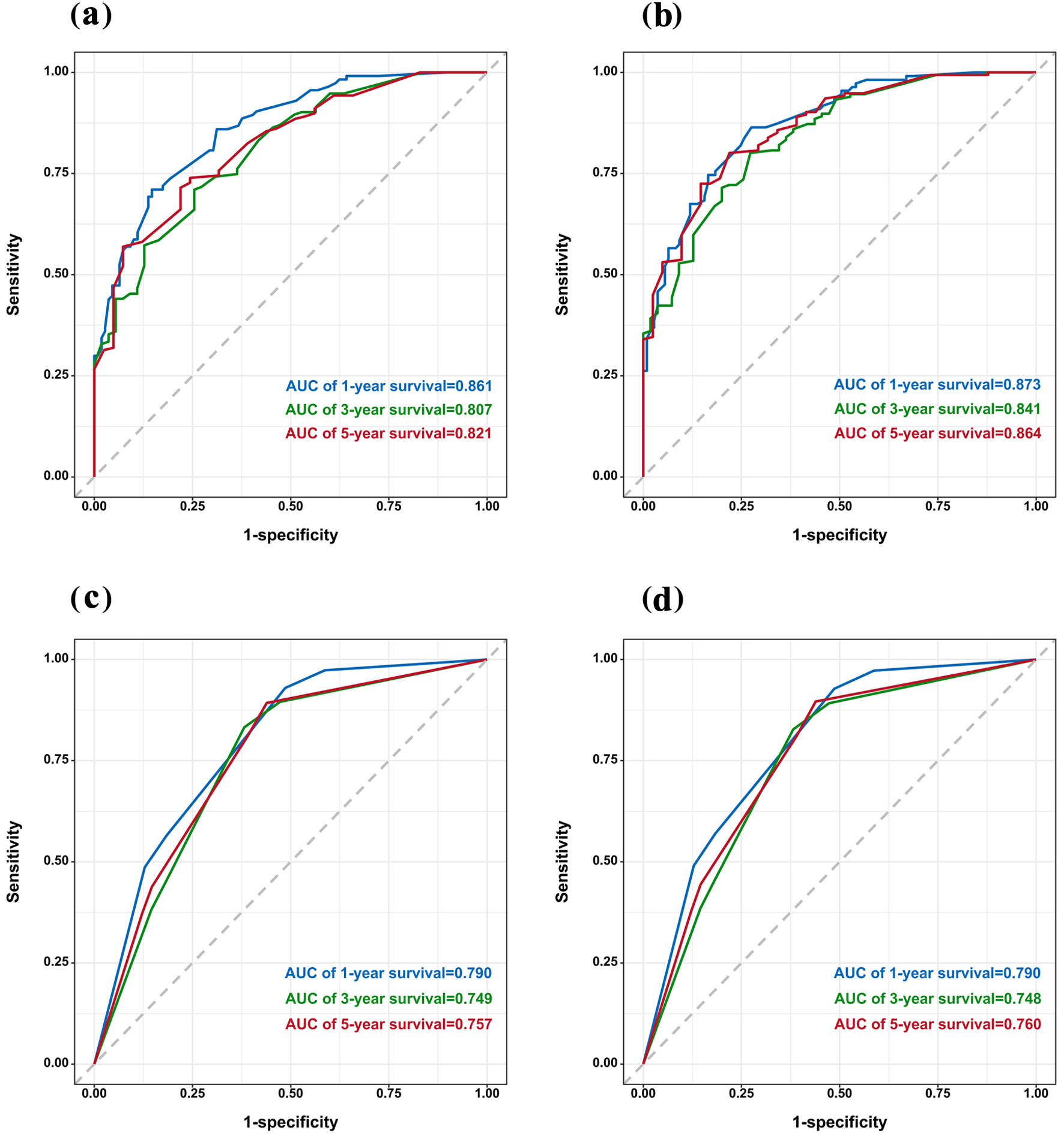 Click for large image | Figure 3. ROC curve of the nomogram (a) and FIGO stage (c) for OS. ROC curve of the nomogram (b) and FIGO stage (d) for CSS. CSS: cancer-specific survival; FIGO: Federation International of Gynecology and Obstetrics; OS: overall survival; ROC: receiver operating characteristic. |
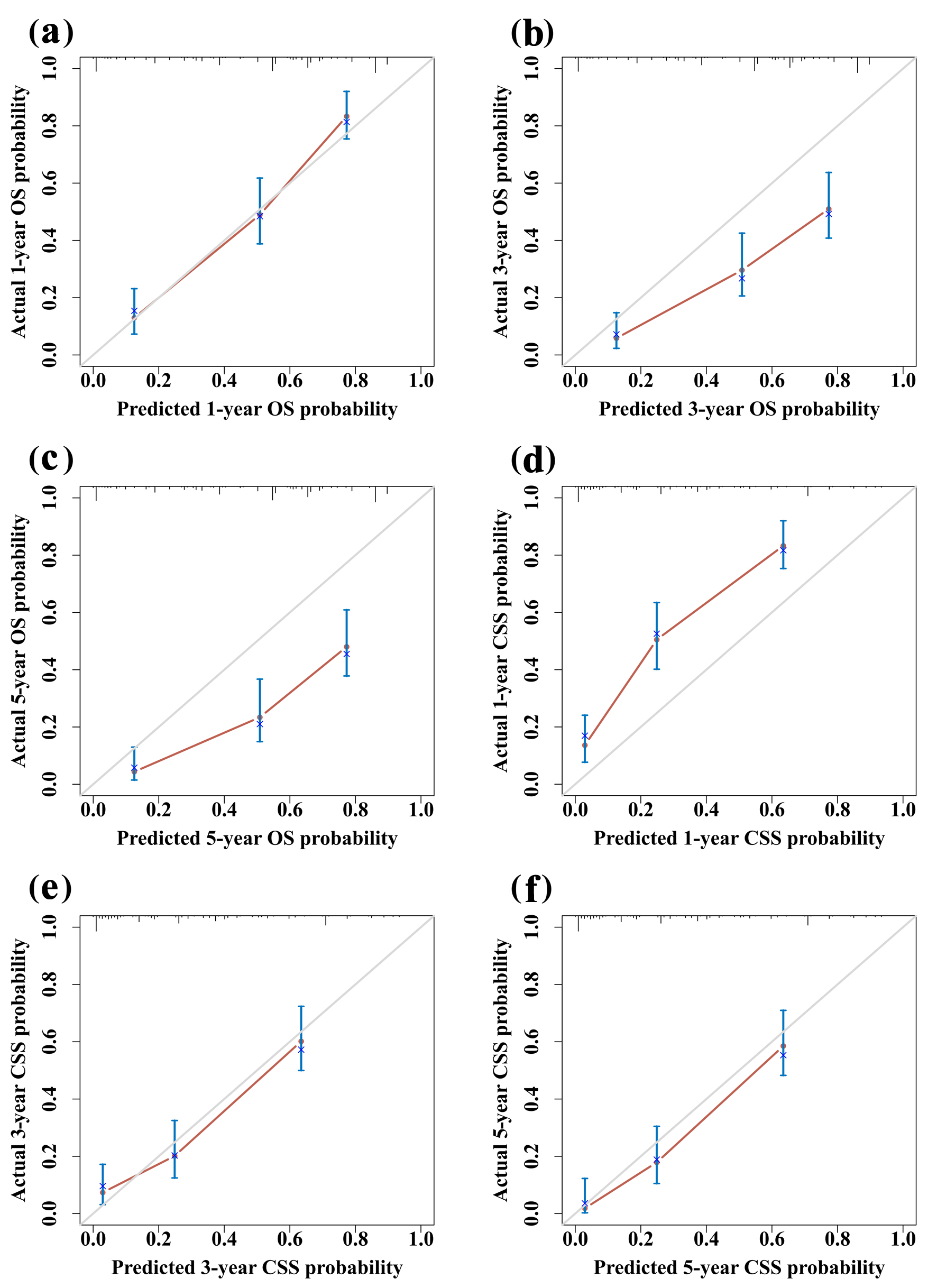 Click for large image | Figure 4. Calibration curves for 1-year (a), 3-year (b), and 5-year (c) OS. Calibration curves for 1-year (d), 3-year (e), and 5-year (f) CSS. CSS: cancer-specific survival; OS: overall survival. |
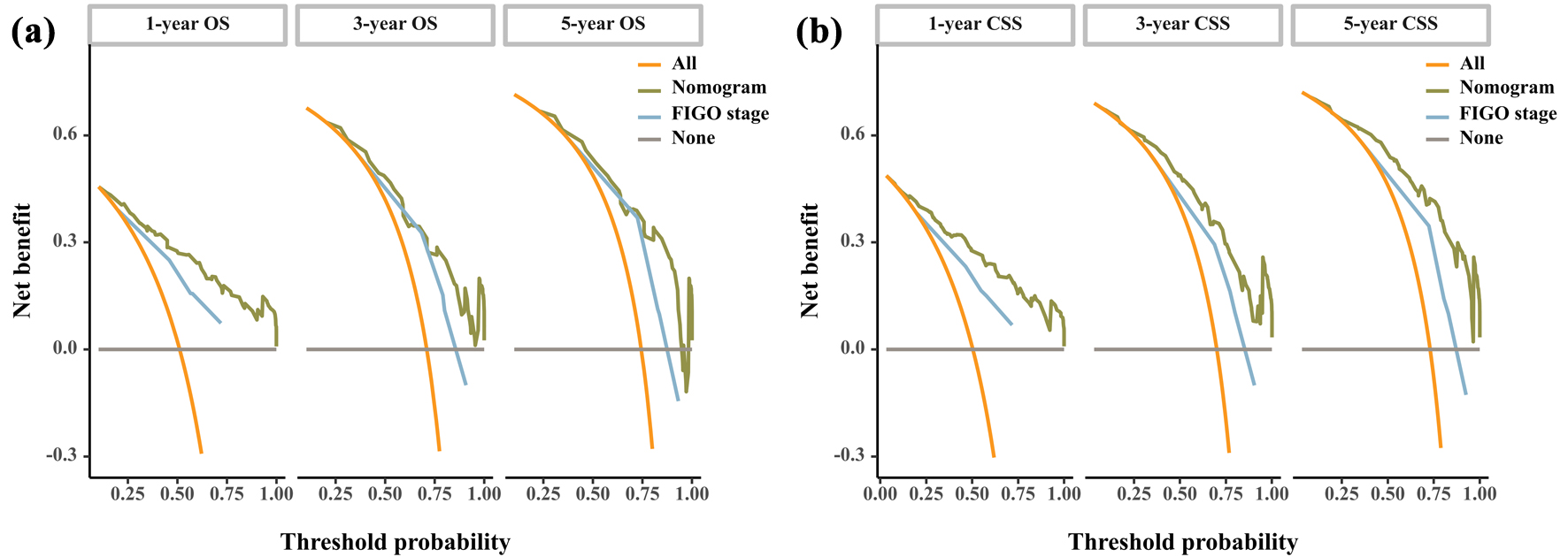 Click for large image | Figure 5. DCA curve of the nomogram and FIGO stage for OS (a) and CSS (b). CSS: cancer-specific survival; DCA: decision curve analysis; FIGO: Federation International of Gynecology and Obstetrics; OS: overall survival. |
| Discussion | ▴Top |
In the current study, we developed nomograms to predict 1-, 3-, and 5-year OS and CSS for SCCO patients. The calibration and identification of the nomograms were evaluated and these nomograms have promising applications. In particular, the nomograms integrate demographic, clinical, and pathological information and their interactions that affect survival. The accuracy of the prognostic nomogram in predicting SCCO was better than the current FIGO staging system, based on the ROC and DCA curves. Critically, the nomograms provide personalized, patient-specific estimates of OS and CSS that can be used for risk stratification and prognostic discussion in patients with SCCO.
Current SEER database-driven investigations on SCCO remain scarce, exemplified by Jamy et al’s comparative analyses with small cell lung cancer [18], Wang et al’s lymphadenectomy evaluations [19], and Hu et al’s nomograms validated solely by single metrics [20]. In contrast, our study developed prognostic nomograms for predicting OS and CSS in SCCO patients, systematically evaluated through the C-index, AUC, calibration curves, and DCA, thereby demonstrating clinical utility. A previous study has shown that age is an independent factor affecting the prognosis of SCCO patients, and the older the age, the worse the prognosis [21]. In addition, it has been reported that median household income, laterality, and the number of primary tumors are factors for a better prognosis [20]. Our results confirmed that age ≥ 73 years, median household income > $55,000, and only one primary tumor were risk factors for SCCO. Early diagnosis plays a crucial role in the optimal treatment outcome of ovarian tumors, including SCCO. Serum CA125 is now widely accepted as an important prognostic factor for OS and progression-free survival in ovarian cancer [8]. A systematic review reported that 80% of SCCOHT patients had elevated serum CA125 levels, but little is known about the prognostic value of CA125 in SCCOHT [8]. Our study found that CA125 did not determine the prognosis of SCCO patients, probably because changes in CA125 were not related to surgery/radiotherapy/chemotherapy status. Therefore, further longitudinal survey data are needed to confirm whether CA125 can be used to predict prognosis in SCCO.
In addition to age, median household income, and the number of primary tumors, we also showed that surgery status, chemotherapy, and radiotherapy were independent risk factors for SCCO patients. Among them, the FIGO staging system is the most commonly used staging system for SCCO: the higher the stage, the lower the survival rate. Although various treatment approaches have been proposed for primary SCCO, there is no international consensus on surveillance and therapeutic strategy [5]. Surgical treatment supplemented with multi-drug combination chemotherapy and radiotherapy is the current main treatment strategy [5]. The basic surgical procedures include total hysterectomy and bilateral adnexectomy resection, retroperitoneal lymph node dissection, pelvic and abdominal implant foci debulking, pelvic and para-aortic lymphadenectomy, etc. Several research groups have found that SCCOHT is characterized by germline and somatic deleterious mutations (henceforth termed pathogenic variants (PVs)) in SMARCA4 [22]. Fertility-sparing surgery may be considered in stage IA SCCOHT patients without germline SMARCA4 PVs who wish to have future pregnancies [6]. There is no standard chemotherapy regimen for patients with SCCO, and most are generally based on a combination of cisplatin and etoposide. Baeyens et al found that a 17-year-old girl with stage IA SCCO survived for 10 years after radiotherapy, but whether this indicates that radiotherapy is effective for early SCCO needs to be further confirmed [23]. In conclusion, the choice of surgical approaches and radiotherapy and chemotherapy regimens for primary SCCO needs to be further explored on the basis of the existing evidence.
Currently, the FIGO staging system is mainly used for ovarian cancer, which is an important independent influencing factor. The higher the stage, the lower the survival rate. In this study, Cox multifactorial analysis showed that the FIGO stage was an independent factor affecting the prognosis of patients with SCCO, which was consistent with previous reports in the literature [20]. However, the FIGO staging system ignores other factors such as tumor size, histological grade, treatment, age, income, and ethnicity, and there are limitations in predicting the prognosis of SCCO based on the FIGO staging system alone. Thus, we developed nomograms for predicting survival in patients with SCCO that contain more comprehensive predictors. In this study, by comparing the C-index, ROC curves, and DCA curves, we found that the nomograms model predicted patient prognosis more accurately than the FIGO staging system and that the patients assessed had greater clinical benefit. The nomograms could be used for risk stratification and prognostic discussions in patients with SCCO, thus contributing to individualized treatment regimens and follow-up plans.
In this study, we constructed nomograms based on the results of multivariate Cox regression analyses and incorporated common clinical factors and demographic information into the models. Our nomograms predicted OS and CSS well, and their predictions were supported by bootstrap-corrected C-index, calibration curves, ROC curves, and DCA curves. However, some limitations need to be acknowledged in this study. First, detailed treatment information, such as surgical treatment modalities, specific protocols, doses, and number of cycles of chemotherapy or radiotherapy may have an impact on the patient’s prognosis. However, the SEER database categorizes treatment status only as yes or no/unknown, a design constraint that obscures protocol-specific details and risks outcome bias, as no/unknown groups may include undocumented therapies. Second, given the rarity of SCCO, we could only evaluate nomograms internally. Despite the adequate discrimination and calibration capabilities of our nomograms, as well as their good clinical applicability, external validation of the models in prospective and multi-centric studies with a large sample size is still needed. Third, this study was a retrospective study, and selection bias is inevitable. Fourth, the SEER database exhibits substantial missingness in key prognostic variables such as histological grade, LNS, and CA125 status, which can have an impact on predictive models.
Conclusions
In conclusion, we developed and evaluated new nomograms to predict OS and CSS probability in SCCO patients. The nomograms showed adequate discrimination and calibration capabilities, as well as good clinical applicability, and could be used as a useful tool for clinicians to assess the prognosis of SCCO patients and determine individualized treatment regimens.
| Supplementary Material | ▴Top |
Suppl 1. The International Federation of Gynecology and Obstetrics stages assessing 1-, 3-, and 5-year survival of SCCO patients. (A) Overall survival. (B) Cancer-specific survival. FIGO: Federation International of Gynecology and Obstetrics; SCCO: small cell carcinoma of ovary.
Acknowledgments
We sincerely thank the data support provided by the SEER database.
Financial Disclosure
This study was funded by the Health Industry Foundation of Lanzhou City (A2023008) and the Natural Science Foundation of Gansu Province (25JRRA155).
Conflict of Interest
There is no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Chun Mei Yan and Ya Rong Chen designed the study, analyzed data, and wrote, reviewed, and edited the manuscript. Hong Fang Li and Ri Cheng Li contributed to discussion, and reviewed and edited the manuscript. Chun Mei Yan was the guarantor of this work and, as such, had full access to all the data in the study and took responsibility for the completeness of the data and the accuracy of the data analysis.
Data Availability
The datasets generated during and/or analyzed during the study are available from the corresponding author on a reasonable request.
Abbreviations
AUC: area under the curve; CA125: serum carbohydrate antigen 125; CIs: confidence intervals; C-index: concordance index; CSS: cancer-specific survival; DCA: decision curve analysis; DSW: divorced, separated, or widowed; FIGO: International Federation of Gynecology and Obstetrics; HRs: hazard ratios; ICD-O-3: International Classification of Oncological Diseases 3; LNS: lymph node status; OS: overall survival; SCCO: small cell carcinoma of ovary; SCCOHT: SCCO hypercalcemic type; SCCOPT: SCCO pulmonary type; SEER: Surveillance, Epidemiology, and End Results
| References | ▴Top |
- Reed NS, Pautier P, Avall-Lundqvist E, Choi CH, du Bois A, Friedlander M, Fyles A, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian small cell cancers. Int J Gynecol Cancer. 2014;24(9 Suppl 3):S30-34.
doi pubmed - Li R, Zhou T, Chen S, Li N, Cai Z, Ling Y, Feng Z. Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT): a challenge for clinicopathological diagnosis. Int J Clin Exp Pathol. 2019;12(6):2166-2172.
pubmed - Munstedt K, Estel R, Dreyer T, Kurata A, Benz A. Small cell ovarian carcinomas - characterisation of two rare tumor entities. Geburtshilfe Frauenheilkd. 2013;73(7):698-704.
doi pubmed - Scully RE. Small cell carcinoma of hypercalcemic type. Int J Gynecol Pathol. 1993;12(2):148-152.
doi pubmed - Ray-Coquard I, Morice P, Lorusso D, Prat J, Oaknin A, Pautier P, Colombo N, et al. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv1-iv18.
doi pubmed - Tischkowitz M, Huang S, Banerjee S, Hague J, Hendricks WPD, Huntsman DG, Lang JD, et al. Small-cell carcinoma of the ovary, hypercalcemic type-genetics, new treatment targets, and current management guidelines. Clin Cancer Res. 2020;26(15):3908-3917.
doi pubmed - Lang JD, Hendricks WPD. Identification of driver mutations in rare cancers: the role of SMARCA4 in small cell carcinoma of the ovary, hypercalcemic type (SCCOHT). Methods Mol Biol. 2018;1706:367-379.
doi pubmed - Wens F, Hulsker CCC, Fiocco M, Zsiros J, Smetsers SE, de Krijger RR, van der Steeg AFW, et al. Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT): patient characteristics, treatment, and outcome-a systematic review. Cancers (Basel). 2023;15(15):3794.
doi pubmed - Fahiminiya S, Sabbaghian N, Albrecht S, Nadaf J, Callegaro-Filho D, Foulkes WD. Ovarian small cell carcinoma in one of a pair of monozygous twins. Fam Cancer. 2019;18(2):161-163.
doi pubmed - Simoes MFE, da Costa A, Silva TN, Fernandes L, Bovolim G, Torrezan GT, Carraro DM, et al. Case report of small cell carcinoma of the ovary, hypercalcemic type (Ovarian Rhabdoid Tumor) with SMARCB1 mutation: a literature review of a rare and aggressive condition. Curr Oncol. 2022;29(2):411-422.
doi pubmed - Gupta P, Kapatia G, Gupta N, Dey P, Rohilla M, Gupta A, Rai B, et al. Small cell carcinoma of the ovary: clinicopathologic and immunohistochemical analysis of 7 new cases of a rare malignancy. Int J Surg Pathol. 2021;29(3):236-245.
doi pubmed - Yang P, Qiu J, Li J, Wu D, Wan X, Lau WY, Yuan Y, et al. Nomograms for pre- and postoperative prediction of long-term survival for patients who underwent hepatectomy for multiple hepatocellular carcinomas. Ann Surg. 2016;263(4):778-786.
doi pubmed - Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173-180.
doi pubmed - Wang J, Zhanghuang C, Tan X, Mi T, Liu J, Jin L, Li M, et al. A nomogram for predicting cancer-specific survival of osteosarcoma and Ewing's sarcoma in children: a SEER database analysis. Front Public Health. 2022;10:837506.
doi pubmed - Lee C, Light A, Alaa A, Thurtle D, van der Schaar M, Gnanapragasam VJ. Application of a novel machine learning framework for predicting non-metastatic prostate cancer-specific mortality in men using the Surveillance, Epidemiology, and End Results (SEER) database. Lancet Digit Health. 2021;3(3):e158-e165.
doi pubmed - Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361-387.
doi pubmed - Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565-574.
doi pubmed - Jamy O, Yaghmour G, Hare F, Martin MG. Population-based analysis of the clinical features of primary small cell carcinoma of the ovary. Anticancer Res. 2015;35(5):3091-3095.
pubmed - Wang J, Ning Y, Du Y, Kang Y. Lymphadenectomy benefits small cell carcinoma of ovary: a population-based analysis. Curr Oncol. 2022;29(10):7802-7815.
doi pubmed - Hu D, Ma D, Zhang ZJ, Zhang Y, Huang K, Li X. Prognosis comparison between small cell carcinoma of ovary and high-grade serous ovarian cancer: a retrospective observational cohort study. Front Endocrinol (Lausanne). 2023;14:1103429.
doi pubmed - Nasioudis D, Chapman-Davis E, Frey MK, Caputo TA, Witkin SS, Holcomb K. Small cell carcinoma of the ovary: a rare tumor with a poor prognosis. Int J Gynecol Cancer. 2018;28(5):932-938.
doi pubmed - Lin DI, Chudnovsky Y, Duggan B, Zajchowski D, Greenbowe J, Ross JS, Gay LM, et al. Comprehensive genomic profiling reveals inactivating SMARCA4 mutations and low tumor mutational burden in small cell carcinoma of the ovary, hypercalcemic-type. Gynecol Oncol. 2017;147(3):626-633.
doi pubmed - Baeyens L, Amat S, Vanden Houte K, Vanhoutte P, L'Hermite M. Small cell carcinoma of the ovary successfully treated with radiotherapy only after surgery: case report. Eur J Gynaecol Oncol. 2008;29(5):535-537.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.