| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 1, February 2025, pages 70-82
Effect of Whey Protein Supplementation on Postoperative Outcomes After Gynecological Cancer Surgery: A Randomized Controlled Trial
Wiranchana Chittia , Putsarat Insina, b, c
, Nisa Prueksaritanonda, b
aDepartment of Obstetrics and Gynecology, Rajavithi Hospital, Bangkok, Thailand
bCollege of Medicine, Rangsit University, Bangkok, Thailand
cCorresponding Author: Putsarat Insin, Department of Obstetrics and Gynecology, Rajavithi Hospital, Bangkok, Thailand
Manuscript submitted October 15, 2024, accepted December 27, 2024, published online January 2, 2025
Short title: WPS and Postoperative Outcomes
doi: https://doi.org/10.14740/wjon1990
| Abstract | ▴Top |
Background: Whey protein’s biochemical properties make it an ideal nutritional supplement for patients with cancer, especially in perioperative care. Thus, the present study aims to assess the efficacy of whey protein supplementation (WPS) compared to standard care in enhancing postoperative outcomes for patients undergoing comprehensive surgical staging for gynecological cancer.
Methods: In an open-label, randomized controlled trial conducted at Rajavithi Hospital between November 28, 2023 and July 8, 2024, 61 patients scheduled for comprehensive surgical staging were enrolled. Participants were randomized in a 1:1 ratio to either the WPS group (n = 30) or the control group (n = 31). The WPS group received isolated whey protein powder (20 g of protein per serving), administered at 6 pm before surgery and 6 am on the first postoperative day. The control group received standard postoperative care. The primary endpoint was the length of hospital stay (LOHS), with secondary outcomes including gastrointestinal function recovery, postoperative analgesic use, complications, and potential WPS-related adverse events such as transaminitis, acute kidney injury, and electrolyte imbalances.
Results: The WPS group had a significantly shorter LOHS than the control group (79.0 ± 6.7 vs. 93.3 ± 28.4 h, P = 0.021). Additionally, the WPS group demonstrated significant improvements in gastrointestinal function, with shorter times to first flatus (P < 0.001), first defecation (P = 0.013), and first ambulation (P = 0.043). No significant differences were observed between the groups regarding postoperative analgesic use or complications, including fever, nausea/vomiting, wound infection, and readmission (P > 0.05). Furthermore, no WPS-related adverse events were reported.
Conclusion: The use of WPS in the perioperative operative management of gynecological cancer surgery yields promising results by significantly reducing the LOHS and accelerating the recovery of gastrointestinal function while maintaining a favorable safety profile.
Keywords: Whey protein supplementation; Gynecological cancer surgery; Postoperative outcomes; Randomized controlled trial
| Introduction | ▴Top |
Gynecological cancers, including cervical, ovarian, uterine, vaginal, and vulvar cancers, are the second most common malignancy affecting the female reproductive system after breast cancer. In 2023, approximately 114,810 new cases of gynecological cancers were diagnosed worldwide, with 34,020 associated deaths [1]. Surgical staging remains a key treatment modality, particularly for ovarian, uterine, and vulvar cancers. The approach typically involves the removal of the primary tumor and evaluation of both local and distant spread, often requiring total abdominal hysterectomy, bilateral salpingo-oophorectomy, and lymphadenectomy. These extensive procedures, particularly in advanced cases, lead to longer hospital stays and increased morbidities.
Postoperative complications, such as fever, respiratory infection, surgical site infection, urinary tract infection, bowel ileus, and venous thromboembolism, can arise at any stage after surgery. The incidence of major complications is 3.7% for general gynecological surgeries, but this rate increases to 9.8% for gynecological cancer surgeries. Previous research showed that gynecological cancer procedures carry a 1.6-fold higher risk of significant morbidity compared to benign gynecological procedures [2].
Major complications often increase resource consumption (e.g., laboratory tests, imaging investigation) and may necessitate invasive interventions, such as percutaneous drainage, reoperations, or admission to the intensive care unit (ICU). In severe cases, complications may even result in death. Beyond causing significant stress to patients, these complications can severely diminish the quality of life and delay necessary adjuvant treatments, ultimately affecting survival rates [3]. Moreover, the repercussions extend to patients’ families, imposing emotional strain and significantly raising healthcare costs [4]. Thus, the physical, psychological, and financial burdens of postoperative complications are widely recognized, making them a focal point in the ongoing efforts to enhance the quality and efficiency of gynecological cancer care.
The Enhanced Recovery After Surgery (ERAS) protocol is a comprehensive, evidence-based approach designed to optimize postoperative recovery and reduce morbidity. Initially developed in Europe and later adopted by colorectal surgeons in the United States, its success in improving patient outcomes has led to its expansion across nearly all major surgical specialties, including liver, gastric, breast, esophageal, orthopedic, pancreatic, and urological procedures [5]. Also, ERAS guidelines were first introduced in gynecologic oncology in 2016, with updates in 2019 and 2023, offering substantial benefits such as reduced surgical complications, shorter hospital stays, lower healthcare costs, and improved patient quality of life [6-9]. Despite these advantages, the complexity of managing pre-, intra-, and postoperative phases, along with the need for coordinated efforts among various healthcare professionals, presents significant challenges in clinical implementation. As a result, simplified but effective therapeutic approaches have been developed and integrated into standard care for gynecological cancer surgery.
Malnutrition and cancer cachexia are common and significant challenges in cancer care, closely associated with adverse clinical outcomes, including a higher risk of postoperative complications, prolonged hospital stays, increased vulnerability to chemotherapy-related toxicity, diminished treatment effectiveness, lower quality of life, and decreased survival rates [10]. Malnutrition is particularly common among patients with gynecological cancers, especially those with ovarian cancer [11]. Serum albumin concentration has long been regarded as a reliable marker of malnutrition, with hypoalbuminemia identified as a major risk factor for poor perioperative outcomes and reduced survival rates in gynecological cancer patients [12-14]. Additionally, albumin levels typically decline sharply after major abdominal surgery, reflecting the body’s acute stress response, which further exacerbates negative clinical outcomes [15].
As a result, the 2019 updated ERAS guidelines recommended a high protein diet of 2.0 grams (g) per kilogram (kg) per day in the postoperative care of surgical patients [8]. This approach has been shown to accelerate discharge and reduce complications by mitigating surgical stress, reducing inflammation, and promoting improved postoperative healing [16]. Furthermore, increased protein intake supports protein balance and helps prevent muscle protein degradation during recovery and periods of immobilization [17].
Whey protein, or lactoserum, is the yellow-green liquid byproduct of casein coagulation in milk, comprising 20% of bovine milk’s protein, while the remaining 80% is casein [18]. The primary components of whey proteins include β-lactoglobulin (50-55%), α-lactalbumin (20-25%), immunoglobulins (10-15%), and bovine serum albumin (5-10%), along with minor proteins such as lactoferrin, lactoperoxidase, glycomacropeptide, protease-peptone, and osteopontin. These components provide various health benefits, such as immune modulation, anti-cancer, antimicrobial, anti-inflammatory, cardioprotective, and neuroprotective effects [18, 19]. In addition, whey protein is rich in branched-chain amino acids, such as leucine, isoleucine, and valine, as well as essential amino acids (e.g., cysteine) and peptides. Leucine, present in 50-75% higher concentrations in whey than in other protein sources, plays a crucial role in muscle protein synthesis and minimizing muscle protein breakdown. Meanwhile, cysteine, a precursor to glutathione, helps reduce oxidative stress and regulate cellular processes [18]. Given these properties, whey protein is a promising dietary approach for perioperative nutrition, supporting postoperative recovery and reducing morbidity.
Whey proteins are available in three distinct derivatives based on processing techniques and compositions: whey protein concentrate, isolate, and hydrolysate [18, 19]. Whey protein concentrate contains 34-89% protein, along with fat and lactose, making it higher in calories. Whey protein isolate, with at least 90% protein and minimal lactose or fat, is ideal for those with lactose intolerance. Whey protein hydrolysates, derived from concentrates or isolates through acids, enzymes, or heat, consist of smaller peptides and amino acids, facilitating better absorption in the digestive system. With its lactose- and fat-free composition, along with its high protein quality and content, whey protein isolate is particularly suited for cancer patients. Previous research indicated that supplementation with whey protein isolates during chemotherapy improves nutritional status and immune function [20]. Consequently, whey protein isolate is drawing attention to its potential to enhance surgical outcomes in oncology.
The role of whey protein supplementation (WPS) in perioperative nutritional care gynecological cancer surgery remains under-researched. A randomized controlled trial (RCT) demonstrated that combining whey protein with carbohydrate loading significantly improved postoperative outcomes in terms of shortened hospital stays and increased muscle mass and strength [21]. Building on this evidence, we designed an RCT to assess the efficacy of WPS in gynecological cancer patients undergoing comprehensive surgical staging surgery. This study aims to determine whether perioperative WPS enhances postoperative recovery without adverse effects, specifically hypothesis testing if WPS reduces the length of hospital stays, accelerates bowel function recovery, and lowers postoperative morbidities.
| Materials and Methods | ▴Top |
Study design and settings
This open-label RCT included 74 patients who underwent elective comprehensive surgical staging for gynecological cancers between November 28, 2023 and July 8, 2024, at the Department of Obstetrics and Gynecology, Rajavithi Hospital, Bangkok, Thailand. The trial was registered at ClinicalTrial.gov (Registration No. NCT06035510) and received ethical approval from the Institutional Review Board (IRB) of Rajavithi Hospital (Registration No. 66063). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Randomization, concealment, and blinding
After IRB approval, eligible patients were invited to participate upon admission and enrolled after providing written informed consent. Randomization was performed using a web-based block-of-four method by an independent investigator, assigning patients in a 1:1 ratio to receive either WPS (intervention group) or standard care (control group). Randomization numbers were stored in sequentially numbered, sealed opaque envelopes. After surgery, interventions were administered by nursing staff at the gynecologic service ward. Although complete blinding of patients and nursing staff was not feasible, confidentially regarding group assignments was maintained. Accordingly, clinicians, outcome assessors, and investigators remained blinded throughout the study.
Participants
Patients diagnosed with ovarian, fallopian tube, peritoneal, or uterine cancer and scheduled for elective comprehensive surgical staging were recruited. Eligible participants were females aged 18 to 70 years, good consciousness, and able to communicate in Thai. Exclusion criteria included allergies to proteins or dairy products (e.g., cow’s milk, cheese, yogurt, or eggs), pre-existing conditions such as hypertension, diabetes, chronic kidney disease or liver disease, heart disease, or medications including non-steroidal anti-inflammatory drugs (NSAIDs), bisphosphonates, levodopa, tetracycline, and quinolone. Other exclusions included current protein supplementation, pregnancy or breastfeeding, refusal of surgery, expected severe adhesion, anticipated complex procedures involving abdominal visceral organs surgeries (e.g., bowel resection, hepatectomy, splenectomy, or nephrectomy), or suspected benign disease upon intraoperative evaluation.
Intervention
Patients in the intervention group received an oral solution of 23 g whey protein isolate powder (two sachets, each providing 10 g protein, 41 kcal, 0.12 g fat, ≤ 0.12 g carbohydrates, and 0.16 g sodium) mixed in 350 mL of water. This was administered at 6:00 pm the evening before surgery and again at 6:00 am on the first postoperative day, providing each patient with a total of 40 g of protein. Control group patients were instructed to avoid protein supplements and received standard pre- and postoperative care. The whey protein isolate used in this study was Fresubin® Protein powder (Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany), containing 98.7% whey protein isolates, 1.3% soya lecithins, and an emulsifier.
Study procedures
All eligible patients provided written informed consent, and baseline characteristics such as body mass index (BMI), underlying disease, and tumor characteristics were documented. Nutritional status was evaluated using the Rajavithi Subjective Global Assessment (RJ-SGA) tool at the time of admission, and serum albumin levels were measured 48 h before surgery and again on the third postoperative day. RJ-SGA scores were classified as follows: 0 - 5 (normal nutrition), 6 - 12 (malnutrition level 1), 13 - 19 (malnutrition level 2), and 20 - 25 (malnutrition level 3). Hypoalbuminemia was defined as serum albumin level below 3.5 g/L. Patients identified with malnutrition or hypoalbuminemia were referred to a nutritionist for supplementation before discharge.
A standard pre-, peri- and postoperative management protocol was applied to all patients. On the day before surgery, patients received a clear liquid diet and bowel preparation. Prophylactic antibiotics were administrated at anesthesia induction. Consultant anesthesiologists provided general anesthesia, with or without epidural anesthesia. Gynecological oncologists performed the surgeries, with incisions and procedures tailored to each patient, adhering to standard gynecological cancer staging protocols. The postoperative protocol included standard pain medication and anti-emetic. Patients received 2,000 mL of intravenous fluid within the first 24 h. The nasogastric tube was removed after surgery, and the urinary catheter was removed the next morning. Regular oral paracetamol was administered, with additional opioids, NSAIDs, or anti-emetics as needed. All additional medications were recorded. Early mobilization was encouraged 24 h after surgery, starting with 10 min of sitting to prevent hypotension.
The postoperative feeding regimen was standardized for all patients. On the first postoperative day, patients began with 30 - 60 mL of water, gradually increasing to at least 1 L/day until passing flatus. Afterward, they were allowed clear liquids, followed by a soft and then regular diet as tolerated. The WPS group received 23 g of whey protein isolate solution at 6:00 pm before surgery and 6:00 am on the first postoperative day. The nursing staff ensured complete intake by closely monitoring participants until the whey protein isolate solution was fully consumed. Control group patients were kept nothing by mouth following the same feeding protocol until bowel function returned (defined by the passage of flatus without vomiting or abdominal distention). A blinded outcome assessor, an obstetrics and gynecology resident in training, monitored bowel sounds three times daily using a stethoscope, starting 24 h after surgery. Patients were also instructed to notify nurses immediately upon the first passage of flatus, bowel movement, or defecation.
Patients unable to tolerate their diet were placed on nothing by mouth and provided intravenous hydration until symptoms resolved. Nasogastric tubes were used in case of persistent nausea, vomiting, or abdominal distention. Postoperative complications, including hemorrhage, infection, and deep vein thrombosis, were closely monitored. Discharge criteria included stable vital signs without fever for at least 24 h, independent ambulation, tolerance of a regular diet without vomiting, normal urination, flatus and defecation, well-controlled pain, and the absence of postoperative complications.
Outcome measurement
The primary outcome of this study was the length of hospital stay (LOHS), measured from the end of the surgery (defined as 0 h) until the patient met discharge criteria. Secondary outcomes included the time to first bowel sounds, first flatus, first defecation, first ambulation, additional analgesic use, and postoperative complications (e.g., fever, wound infection on postoperative days 7 and 14, and readmission within 30 days). The time to first bowel sounds was measured from the end of surgery to the first bowel sound heard during routine postoperative care, while the time to first ambulation was measured from the end of surgery until the patient could ambulate without assistance.
Potential adverse events related to WPS, such as allergic reactions, gastrointestinal symptoms (e.g., nausea/vomiting, bloating, diarrhea), headache, decreased appetite, acute transaminitis, acute kidney injury, and electrolyte imbalances, were also evaluated at 3 days and 2 weeks after surgery.
Sample size calculation
The sample size calculation for the primary outcome (LOHS) was based on data from Yi et al’s study and the formula for comparing two independent means [21]. In their study, the mean LOHS was 78.13 ± 33.05 h for the whey protein-infused carbohydrate loading group and 99.49 ± 22.54 h for the control group. Using a 0.05 alpha level and 80% power, it was determined that 28 patients per group were required. After adjusting for a potential 30% dropout rate, the final sample size was set at a minimum of 37 patients per group.
Statistical analysis
Statistical analysis was performed using Stata version 15.1 (Stata Corp, College Station, Texas, USA). The effect of WPS was evaluated based on the intention-to-treat principle to maintain the randomization integrity, with patients analyzed in their originally assigned groups. Descriptive statistics for normally distributed continuous variables were analyzed using the Student’s t-test and reported as mean ± standard deviation, while non-normally distributed variables were analyzed with the Mann-Whitney U test and presented as median and range. Categorical variables were assessed using Pearson’s Chi-square or Fisher’s exact test and presented as frequencies and percentages. A generalized linear mixed model (GLMM) was applied to assess pre- and postoperative serum albumin levels and hypoalbuminemia rates, accounting for treatment effects at each time point. Kaplan-Meier methods were used to analyze LOHS, time to first bowel sound, time to first flatus, time to first defecation, and time to first ambulation, with log-rank tests used for group comparisons. Additionally, mean, or median differences with corresponding 95% confidence intervals (95% CIs) were calculated for the primary outcome (LOHS) and secondary outcomes, offering additional context to enhance the interpretation of the findings. Statistical significance was set at a two-sided P-value < 0.05.
| Results | ▴Top |
The consort flow diagram of participants in the study, including the reasons for exclusion, is depicted in Figure 1. Between November 28, 2023 and July 8, 2024, a total of 74 gynecological cancer patients scheduled for elective comprehensive surgical staging at Rajavithi Hospital were enrolled and assessed for eligibility. A total of 101 were initially excluded before randomization: 85 due to meeting the exclusion criteria, two with cow’s milk allergy, one patient denied surgery, and 13 refused to participate. The remaining 74 patients were randomized equally into two groups, with 37 in the WPS group and 37 in the control group. Post-randomization, 13 patients were excluded due to no longer fulfilling the inclusion criteria, suspected benign disease after intraoperative evaluation, or adjacent organ injury during surgery (seven in the WPS group, six in the control group). The final intention-to-treat analysis included 30 patients in the WPS group and 31 in the control group.
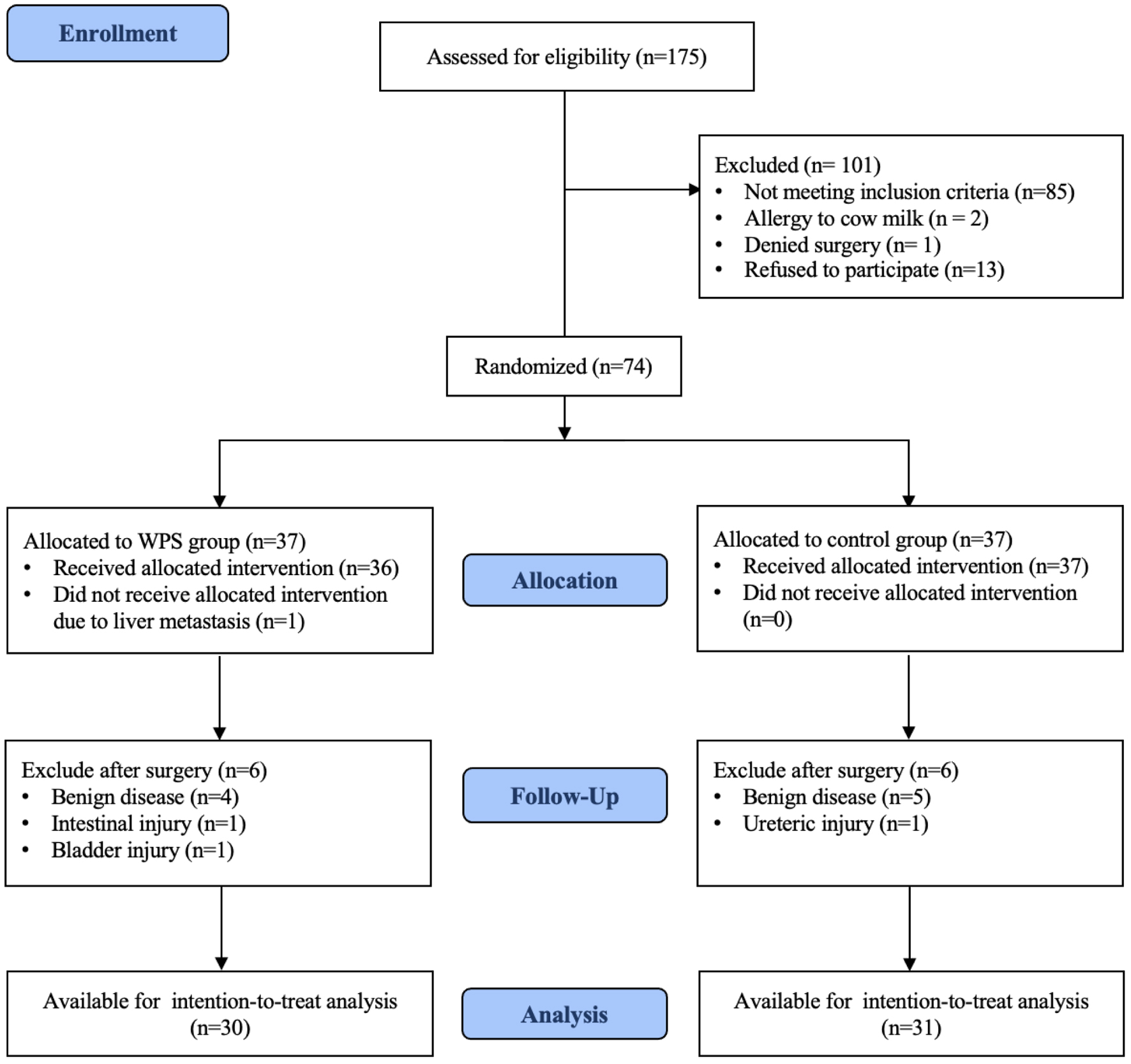 Click for large image | Figure 1. Participant flow diagram after randomization to either whey protein supplementation (WPS) or control group. |
The baseline clinical characteristics of the patients in both groups were similar, as presented in Table 1. The mean age was 51.0 ± 11.8 years, with 60.7% of the participants being postmenopausal. The mean BMI was 25.2 ± 6.5 kg/m2, suggesting that the patient population was generally overweight. Preexisting comorbidities included conditions such as dyslipidemia, asthma, allergic rhinitis, thyroid disease, dissociative disorders, and a history of pulmonary tuberculosis. Additionally, 35% of patients had undergone prior surgeries, and only one patient (1.6%) was a smoker. In terms of baseline nutritional status, all patients were classified as malnourished, with 82% categorized as malnutrition level 1 and 18% as malnutrition level 2. However, both groups were well-balanced in these characteristics.
 Click to view | Table 1. Baseline Clinical Characteristics of Participants in WPS Group and Control Group |
The surgical characteristics between the WPS group and control group, as summarized in Table 2, revealed no statistically significant differences. The most common preoperative diagnosis was suspected ovarian cancer, representing 55.7% of the total cases. A larger percentage of patients in the WPS group, however, were suspected of uterine cancer (56.7%), which correlated with a higher incidence of endometrial cancer, while ovarian cancer was more common in the control group. The most common surgical procedure performed was total abdominal hysterectomy with either unilateral or bilateral salpingo-oophorectomy. Additionally, lymphadenectomy was performed in 40.7% of the patients. Although there was a trend toward reduced estimated blood loss and fewer packed red cell transfusions in the WPS group compared to the control group, these differences were not statistically significant.
 Click to view | Table 2. Surgical Characteristics of Participants in WPS Group and Control Group |
Table 3 compares pre- and postoperative serum albumin levels and hypoalbuminemia rates between the WPS and control groups using GLMM analysis. After adjusting for period and treatment effect, the WPS group had significantly higher serum albumin levels than the control group (mean difference 0.24 g/L; 95% CI: 0.02 to 0.46; P = 0.031). Although both groups experienced a postoperative decrease in serum albumin levels, the WPS group still maintained significantly higher levels compared to the control group (mean difference 0.27 g/L; 95% CI: 0.03 to 0.51; P = 0.026). Moreover, the risk of hypoalbuminemia was 62% lower in the WPS group than in the control group (odds ratio 0.38; 95% CI: 0.18 to 0.80; P = 0.011).
 Click to view | Table 3. Comparison of Pre- and Postoperative Serum Albumin Among WPS Group and Control Group |
WPS was well tolerated, with all patients in the intervention group completing their intake without experiencing nausea, vomiting, or choking. Furthermore, no adverse events such as allergic reactions, gastrointestinal symptoms, headache, decreased appetite, acute transaminitis, acute kidney injury, or electrolyte imbalances were observed concerning WPS.
We observed a significantly shorter LOHS in the WPS group compared to the control group (79.0 vs. 93.3 h; mean difference -14.3 h; 95% CI: -26.3 to -2.3; P = 0.021), as shown in Table 4. Additionally, the WPS group also had significantly shorter times for the first flatus, first defecation, and first ambulation (P < 0.05). Although the time to the first bowel sound was shorter in the WPS group, the difference was not statistically significant (P > 0.05). These findings are visually represented in the Kaplan-Meier curves in Figures 2 and 3.
 Click to view | Table 4. Postoperative Outcomes Categorized by WPS Group and Control Group |
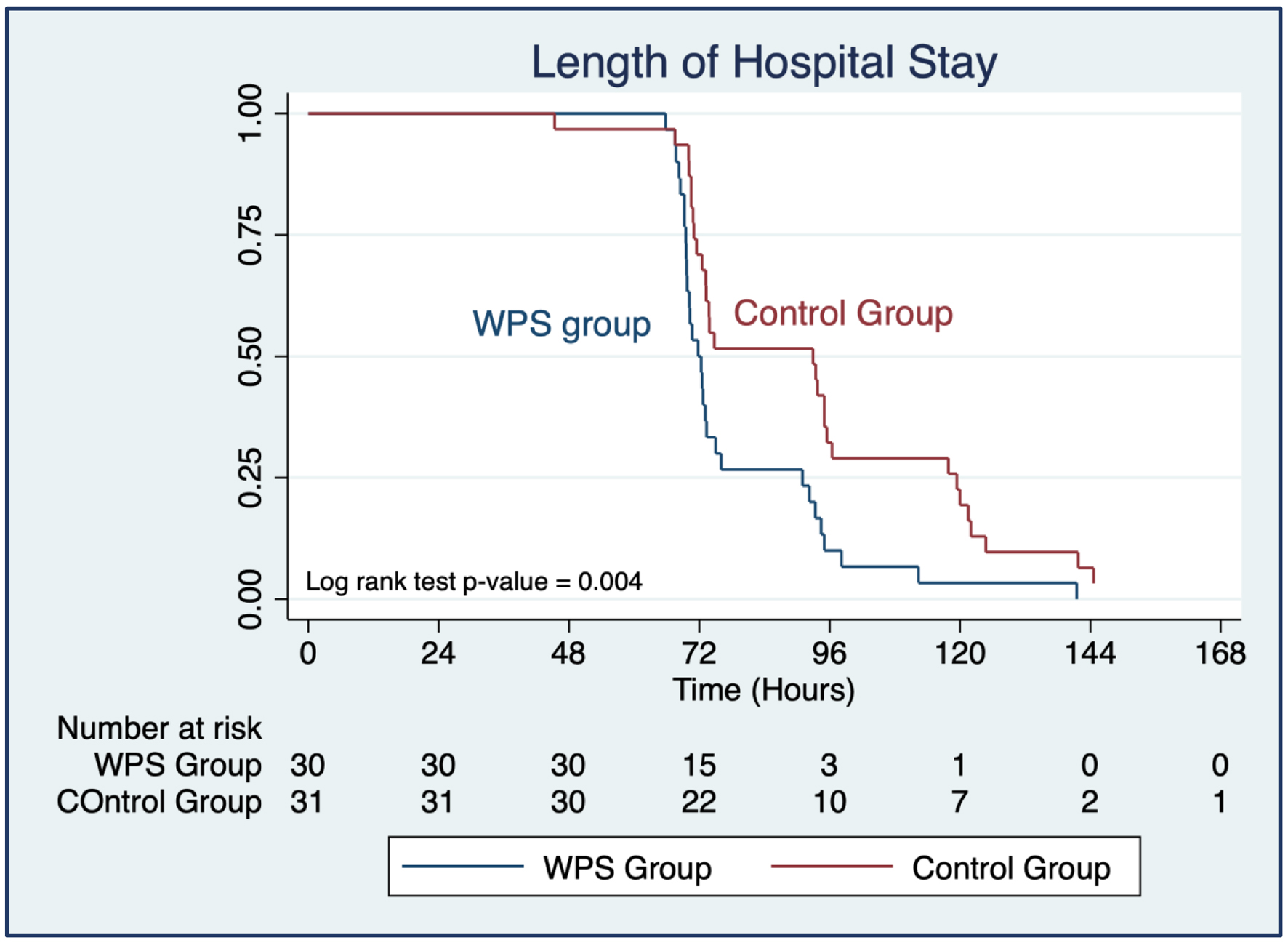 Click for large image | Figure 2. Kaplan-Meier survival estimates for length of hospital stay of participants categorized by whey protein supplementation (WPS) and control group. |
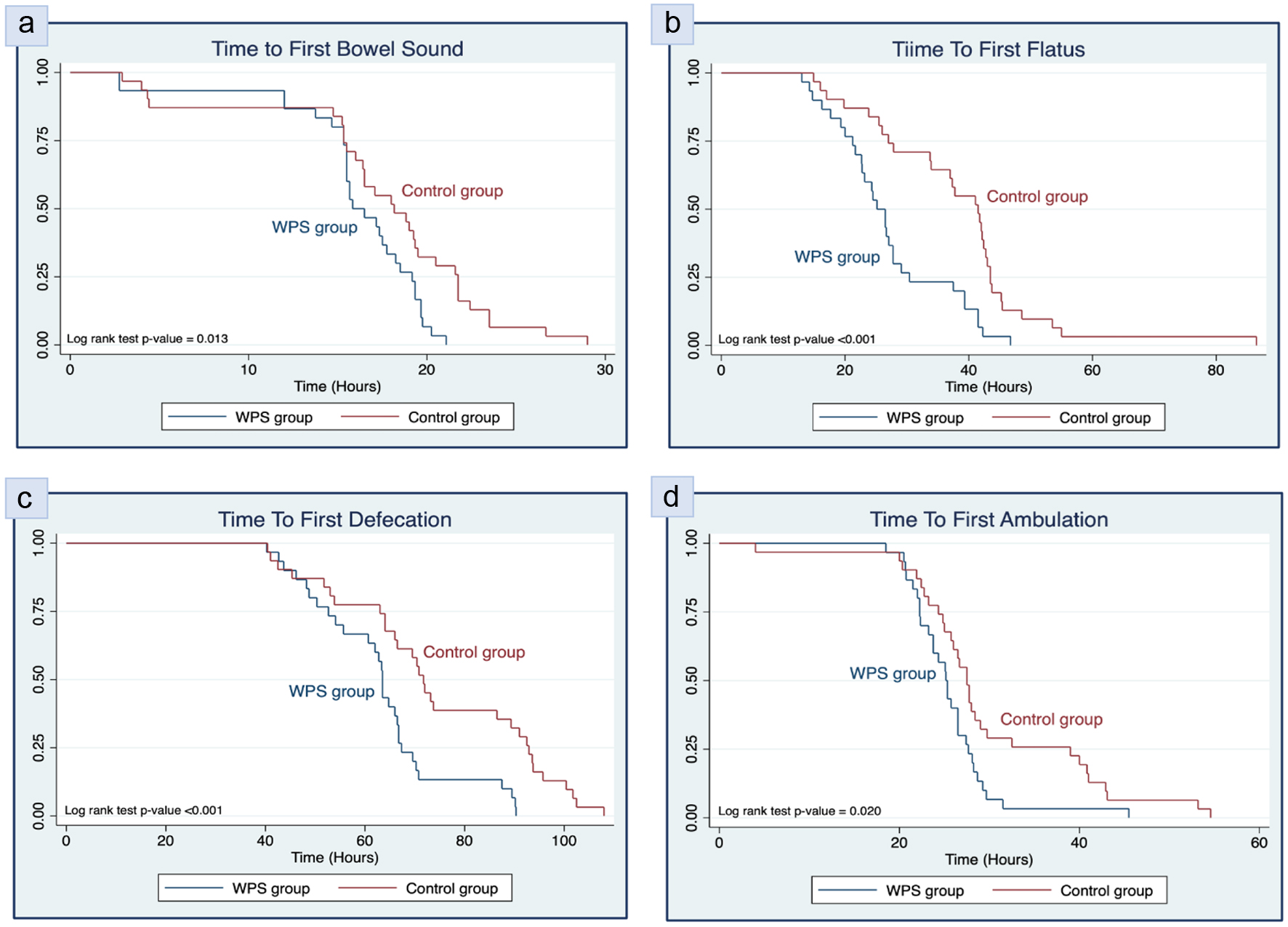 Click for large image | Figure 3. Kaplan-Meier survival estimates for (a) time to first bowel sound, (b) time to first flatus, (c) time to first defecation, and (d) time to first ambulation of participants categorized by whey protein supplementation (WPS) and control group. |
Postoperative outcomes revealed that the WPS group required additional analgesic drugs at a similar rate to the control group, though the trend was lower (6.7% vs. 16.1%, P = 0.425), as shown in Table 4. Postoperative complications between the two groups are detailed in Table 5. The rates of fever, postoperative nausea/vomiting, wound infection, and readmission were comparable between the two groups (P > 0.05).
 Click to view | Table 5. Postoperative Complications Categorized by WPS Group and Control Group |
| Discussion | ▴Top |
Effective perioperative nutrition strategies are essential for promoting faster postoperative recovery and reducing postoperative complications. WPS, a well-known dietary approach, has been widely recognized for its ability to address various conditions such as tumor suppression, prevention of pulmonary infections, enhancement of insulin response, scavenging free radicals, and protection against conditions (e.g., hepatitis, liver fibrosis, and cardiovascular disease), as well as improving post-menopausal well-being [18, 22, 23]. WPS has also been noted for its role in improving perioperative outcomes in cancer patients, showing benefits in reducing LOHS and healthcare costs without increasing complications or readmission. A systemic review and meta-analysis previously demonstrated that WPS improved functional walking capacity before and after cancer surgery, with a reduction in complications such as infections, pneumonia, surgical site infections, and bowel ileus [24]. However, limited research has focused on the role of WPS in the perioperative period for gynecological cancer patients [21]. Thus, this RCT was designed to assess the effectiveness of WPS in shortening LOHS and minimizing postoperative morbidities in patients undergoing gynecological cancer surgery.
In this study, we focused on patients undergoing comprehensive gynecological cancer surgery based on evidence suggesting a 1.6-fold higher risk of major postoperative complications compared to general gynecological surgeries [2]. This elevated risk provides a sufficient basis to identify significant improvements through new measures aimed at reducing these negative outcomes. Regarding intervention of interest, whey protein isolates were selected due to their high-quality protein content, as well as lactose- and fat-free properties, making them ideal and particularly suitable for cancer patients who frequently present with comorbidities such as dyslipidemia and diabetes, and required restricted diet free of fat and sugar which are commonly found in other nutritional supplements. The main goal of incorporating the ERAS program into surgical practice is to promote faster recovery, which is why we chose LOHS as the primary outcome of our study. For the secondary outcomes, we focused on gastrointestinal recovery and postoperative morbidities, which are expected to influence the duration of hospitalization.
The main findings of this study reveal that WPS in the perioperative management of gynecological cancer surgery significantly reduces LOHS and improves gastrointestinal recovery by shortening the time to first flatus, defecation, and ambulation compared to the control group. These clinically relevant results suggest that the WPS offers meaningful benefits in enhancing recovery, minimizing postoperative complications, and lowering healthcare costs in gynecological cancer care.
The primary outcome of this study, LOHS, was significantly reduced in the WPS group compared to the control group, with a mean decrease of 14.3 h. This result is not only statistically significant but also holds clinical importance in the postoperative care of gynecological cancer patients, particularly those with ovarian and endometrial cancers. Many of these patients require subsequent adjuvant therapies, such as chemotherapy, radiotherapy, or both. Delays in recovery and hospital discharge can hinder the timely initiation of these treatments, potentially affecting survival outcomes for gynecological cancer patients [3, 25].
Although not all outcomes showed significant improvement, the results of this study align with previous studies, demonstrating that WPS can accelerate postoperative recovery and reduce LOHS [16, 21, 26]. The improvement observed may be due to the essential role of protein in postoperative recovery, as the surgery increases protein breakdown and raises the body’s protein requirement by 80%, necessitating 1.4 to 2.0 g/kg/day to cope with injury-induced stress [15]. WPS can support muscle synthesis, repair tissue, and reduce inflammation, thus mitigating muscle breakdown, in line with guidelines recommending 20 - 40 g of protein daily for optimal postoperative care in surgical patients [8, 27, 28]. Interestingly, our study’s intervention differed from Yi et al, which combined whey protein with carbohydrate loading, while we focused solely on whey protein isolate [21]. Despite this variation, both studies demonstrated a reduction in LOHS, suggesting that the benefit may primarily arise from protein supplementation rather than carbohydrate loading.
Nevertheless, the findings of this study contrast with earlier research, which showed no significant reduction in LOHS with protein supplementation. Perrone et al found that while preoperative fasting with carbohydrates and whey protein reduced insulin resistance and inflammatory responses, it had no statistical impact on LOHS for patients undergoing cholecystectomy and inguinal herniorrhaphy [29]. Similarly, an RCT by Gillis et al revealed that nutritional counseling with whey protein improved functional walking capacity before colorectal surgery but did not significantly affect postoperative recovery or LOHS [30]. These discrepancies may arise from variations in the type and extent of surgical procedures, as well as several confounding factors influencing LOHS, such as patient’s age, cancer diagnosis, comorbidities, emergency surgeries, and intraoperative or postoperative complications (e.g., adjacent organ injuries, reoperation, postoperative ileus, surgical site infection, pulmonary complications, and cardiac complications) [31].
Previous studies have shown that cancer patients are highly susceptible to malnutrition, which is linked to worse clinical outcomes [10, 11, 32]. In our finding, all patients were found to be malnourished, as determined by the RJ-SGA tools for preoperative nutritional screening, with 82% categorized as level 1 malnutrition and 18% as level 2. This underscores the importance of improving the nutritional status of gynecological cancer patients, both before surgery and prior to starting adjuvant treatment such as chemotherapy or radiotherapy.
Albumin is a well-established marker of protein metabolism, often disrupted following trauma such as surgery, sepsis, or burns. The underlying pathophysiology explanation may involve impaired hepatic albumin synthesis during the early postoperative phase, increased basal energy expenditure, and the consumption of up to 20% of the body’s protein stores. Additionally, capillary leakage, a common feature in sepsis and surgery, leads to albumin sequestration into the third space [15]. Our findings confirm these concepts, as we observed a rapid decline in albumin levels in all postoperative patients.
Notably, serum albumin levels were not only significantly lower in the control group compared to the WPS group, but the WPS group also showed a 62% reduced risk of hypoalbuminemia. These findings are consistent with previous studies highlighting the strong association between perioperative serum albumin levels and adverse outcomes in major abdominal surgeries. A study from Northern Tanzania, which included patients undergoing major abdominal surgery, found that greater albumin declines led to a sixfold higher risk of surgical site infection, delayed wound healing, and increased 30-day mortality (adjusted odds ratio: 6.68; 95% CI: 1.59 to 28.09) [33]. Similarly, research from Thailand identified hypoalbuminemia as an important predictor of delayed bowel function, increased complications, and prolonged LOHS in colon cancer patients [34]. These results suggest that WPS may be an effective intervention to correct hypoalbuminemia and mitigate these negative postoperative outcomes.
In clinical application, WPS should be incorporated into perioperative care guidelines for gynecological cancer surgery, given its association with reducing LOHS by approximately 1 day. This earlier discharge leads to a cost savings of 1,000 Thai Baht (THB) per patient. Furthermore, WPS seems to be cost-effective, costing only 110 THB for a daily intake of 40 g protein (27.5 THB per sachet), compared to the cost related to extended use of medical resources such as laboratory tests, imaging, invasive interventions, reoperations, or ICU admissions. Therefore, a recommended area for future research would be a cost-effectiveness analysis of WPS in improving postoperative outcomes after major abdominal surgery to establish its maximal clinical utility.
The current study presents several strengths, marking the first to demonstrate the positive impact of perioperative nutritional intervention with WPS in enhancing recovery for gynecological cancer surgery patients in Thailand. As a prospective RCT, it effectively minimizes selection bias, ensuring reliable comparisons between the two groups. Both groups exhibited comparable demographic and surgical characteristics, affirming the robustness of the randomization process and suggesting similar patient prognoses. Conducted at a single institution with standardized surgical procedures, this approach reduced variability in techniques and treatment protocols. Additionally, the simplicity, practicality, and high compliance of this intervention make it feasible for implementation in real-world clinical settings.
On the other hand, this study has several limitations. Firstly, the small sample size has limited the detection of significant differences in secondary outcomes, such as time to first bowel sound and postoperative morbidities. However, the study was sufficiently powered to evaluate the effect of WPS on LOHS. Secondly, the study population was highly selective, focusing on healthy patients undergoing comprehensive gynecological cancer surgeries without complications. Complex cases, such as those requiring bowel resection, re-anastomosis, or prolonged postoperative catheterization in cervical cancer and vulvar cancer patients, were excluded. As a result, the safety and efficacy of WPS in high-risk surgeries remain unknown, necessitating further research. Thirdly, the absence of a placebo group introduces potential bias in subjective outcomes such as nausea and vomiting, as patients in the WPS group may have been more motivated due to their treatment. Additionally, using serum albumin levels alone to measure nutritional status might not fully reflect the impact of WPS on the inflammatory response post-surgery. Incorporating markers such as C-reactive protein (CRP) or the CRP-to-albumin ratio could better assess WPS’s effectiveness. The 30-day follow-up period may also have been too short to observe significant postoperative morbidities. Lastly, considering the growing emphasis on patient quality of life, future studies should explore WPS’s role in reducing postoperative symptoms such as thirst, hunger, agitation, satisfaction, and pain relief. These improvements could positively impact the overall quality of perioperative care from the perspectives of surgical patients.
Conclusion
The integration of WPS into the perioperative care of gynecological cancer surgery demonstrates significant promise. It notably reduces LOHS and enhances gastrointestinal recovery while maintaining a favorable safety profile. These results highlight the clinical relevance of nutritional interventions in improving postoperative outcomes. WPS offers a practical and cost-effective supplement to standard perioperative care, showing the potential to enhance recovery even in patients with normal preoperative serum albumin levels by addressing albumin decline following surgery. However, a long-term, multicenter study involving high-risk surgical patients is required to further validate these findings and ensure broader applicability before incorporating WPS into standard clinical guidelines.
Acknowledgments
The authors extend their sincere gratitude to the nurses, obstetrics and gynecology residents in training, and all gynecological oncology staff at the Department of Obstetrics and Gynecology, Rajavithi Hospital, for their invaluable contributions to this study.
Financial Disclosure
This study was supported by the research management fund of Rajavithi Hospital.
Conflict of Interest
The authors declared no conflict of interest, and no specific commercial sponsorship was received from whey protein isolate manufacturers.
Informed consent
Informed consent was obtained from all participants involved in the study.
Author Contributions
Wiranchana Chitti: conceptualization; methodology; data curation; writing - original draft. Putsarat Insin: conceptualization; methodology; formal analysis; supervision; writing - original draft; writing - review and editing. Nisa Prueksaritanond: conceptualization; methodology; writing - review and editing. All authors have reviewed and provided consent for publication.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48.
doi pubmed - Erekson EA, Yip SO, Ciarleglio MM, Fried TR. Postoperative complications after gynecologic surgery. Obstet Gynecol. 2011;118(4):785-793.
doi pubmed - Angeles MA, Hernandez A, Perez-Benavente A, Cabarrou B, Spagnolo E, Rychlik A, Daboussi A, et al. The effect of major postoperative complications on recurrence and long-term survival after cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2022;166(1):8-17.
doi pubmed - Straatman J, Cuesta MA, de Lange-de Klerk ES, van der Peet DL. Hospital cost-analysis of complications after major abdominal surgery. Dig Surg. 2015;32(2):150-156.
doi pubmed - Zhang X, Yang J, Chen X, Du L, Li K, Zhou Y. Enhanced recovery after surgery on multiple clinical outcomes: Umbrella review of systematic reviews and meta-analyses. Medicine (Baltimore). 2020;99(29):e20983.
doi pubmed - Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, Antrobus J, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations—Part I. Gynecol Oncol. 2016;140(2):313-322.
doi pubmed - Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, Antrobus J, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations—Part II. Gynecol Oncol. 2016;140(2):323-332.
doi pubmed - Nelson G, Bakkum-Gamez J, Kalogera E, Glaser G, Altman A, Meyer LA, Taylor JS, et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations-2019 update. Int J Gynecol Cancer. 2019;29(4):651-668.
doi pubmed - Nelson G, Fotopoulou C, Taylor J, Glaser G, Bakkum-Gamez J, Meyer LA, Stone R, et al. Enhanced recovery after surgery (ERAS(R)) society guidelines for gynecologic oncology: Addressing implementation challenges - 2023 update. Gynecol Oncol. 2023;173:58-67.
doi pubmed - Arends J. Malnutrition in cancer patients: Causes, consequences and treatment options. Eur J Surg Oncol. 2024;50(5):107074.
doi pubmed - Laky B, Janda M, Bauer J, Vavra C, Cleghorn G, Obermair A. Malnutrition among gynecological cancer patients. Eur J Clin Nutr 2007;61:642-646.
- Uppal S, Al-Niaimi A, Rice LW, Rose SL, Kushner DM, Spencer RJ, Hartenbach E. Preoperative hypoalbuminemia is an independent predictor of poor perioperative outcomes in women undergoing open surgery for gynecologic malignancies. Gynecol Oncol. 2013;131(2):416-422.
doi pubmed - Ataseven B, du Bois A, Reinthaller A, Traut A, Heitz F, Aust S, Prader S, et al. Pre-operative serum albumin is associated with post-operative complication rate and overall survival in patients with epithelial ovarian cancer undergoing cytoreductive surgery. Gynecol Oncol. 2015;138(3):560-565.
doi pubmed - Seebacher V, Grimm C, Reinthaller A, Heinze G, Tempfer C, Hefler L, Polterauer S. The value of serum albumin as a novel independent marker for prognosis in patients with endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):101-106.
doi pubmed - Hubner M, Mantziari S, Demartines N, Pralong F, Coti-Bertrand P, Schafer M. Postoperative albumin drop is a marker for surgical stress and a predictor for clinical outcome: a pilot study. Gastroenterol Res Pract. 2016;2016:8743187.
doi pubmed - Yeung SE, Hilkewich L, Gillis C, Heine JA, Fenton TR. Protein intakes are associated with reduced length of stay: a comparison between Enhanced Recovery After Surgery (ERAS) and conventional care after elective colorectal surgery. Am J Clin Nutr. 2017;106(1):44-51.
doi pubmed - Smith-Ryan AE, Hirsch KR, Saylor HE, Gould LM, Blue MNM. Nutritional considerations and strategies to facilitate injury recovery and rehabilitation. J Athl Train. 2020;55(9):918-930.
doi pubmed - Min C, Han Y, Liu H, Chen Y, Zhang S, Yao Z, Ding Y. cDNA cloning, recombinant expression and bioactivity of Pere David's deer BAFF. Gene. 2012;505(2):233-239.
doi pubmed - Patel S. Emerging trends in nutraceutical applications of whey protein and its derivatives. J Food Sci Technol. 2015;52(11):6847-6858.
doi pubmed - Bumrungpert A, Pavadhgul P, Nunthanawanich P, Sirikanchanarod A, Adulbhan A. Whey protein supplementation improves nutritional status, glutathione levels, and immune function in cancer patients: a randomized, double-blind controlled trial. J Med Food. 2018;21(6):612-616.
doi pubmed - Yi HC, Ibrahim Z, Abu Zaid Z, Mat Daud Z, Md Yusop NB, Omar J, Mohd Abas MN, et al. Impact of enhanced recovery after surgery with preoperative whey protein-infused carbohydrate loading and postoperative early oral feeding among surgical gynecologic cancer patients: an open-labelled randomized controlled trial. Nutrients. 2020;12(1):264.
doi pubmed - Mehra R, Kumar H, Kumar N, Ranvir S, Jana A, Buttar H, Telessy I, et al. Whey proteins processing and emergent derivatives: an insight perspective from constituents, bioactivities, functionalities to therapeutic applications. Journal of Functional Foods. 2021;87:104760.
doi - Kuo YY, Chang HY, Huang YC, Liu CW. Effect of whey protein supplementation in postmenopausal women: a systematic review and meta-analysis. Nutrients. 2022;14(19):4210.
doi pubmed - Srinivasaraghavan N, Das N, Balakrishnan K, Rajaram S. Effect of whey protein supplementation on perioperative outcomes in patients with cancer - a systematic review and meta-analysis (PROSPERO 2020: CRD42020188666). Nutr Cancer. 2022;74(7):2351-2364.
doi pubmed - Zhao J, Chen R, Zhang Y, Wang Y, Zhu H. Impact of treatment delay on the prognosis of patients with ovarian cancer: a population-based study using the surveillance, epidemiology, and end results database. J Cancer. 2024;15(2):473-483.
doi pubmed - Diaz-Feijoo B, Agusti-Garcia N, Sebio R, Lopez-Hernandez A, Siso M, Glickman A, Carreras-Dieguez N, et al. Feasibility of a multimodal prehabilitation programme in patients undergoing cytoreductive surgery for advanced ovarian cancer: a pilot study. Cancers (Basel). 2022;14(7):1635.
doi pubmed - Blaauw L, Schoonees A, Robertson N, Visser J. The impact of guideline recommended protein intake on mortality and length of intensive care unit and hospital stay in critically ill adults: A systematic review. Clin Nutr ESPEN. 2024;61:356-368.
doi pubmed - Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11-48.
doi pubmed - Perrone F, da-Silva-Filho AC, Adorno IF, Anabuki NT, Leal FS, Colombo T, da Silva BD, et al. Effects of preoperative feeding with a whey protein plus carbohydrate drink on the acute phase response and insulin resistance. A randomized trial. Nutr J. 2011;10:66.
doi pubmed - Gillis C, Loiselle SE, Fiore JF, Jr., Awasthi R, Wykes L, Liberman AS, Stein B, et al. Prehabilitation with whey protein supplementation on perioperative functional exercise capacity in patients undergoing colorectal resection for cancer: a pilot double-blinded randomized placebo-controlled trial. J Acad Nutr Diet. 2016;116(5):802-812.
doi pubmed - Krell RW, Girotti ME, Dimick JB. Extended length of stay after surgery: complications, inefficient practice, or sick patients? JAMA Surg. 2014;149(8):815-820.
doi pubmed - Nasser S, Bilir E, Derin X, Richter R, Grabowski JP, Ali P, Kulbe H, et al. Pre-operative malnutrition in patients with ovarian cancer: what are the clinical implications? Results of a prospective study. Cancers (Basel). 2024;16(3):622.
doi pubmed - Issangya CE, Msuya D, Chilonga K, Herman A, Shao E, Shirima F, Naman E, et al. Perioperative serum albumin as a predictor of adverse outcomes in abdominal surgery: prospective cohort hospital based study in Northern Tanzania. BMC Surg. 2020;20(1):155.
doi pubmed - Lohsiriwat V, Chinswangwatanakul V, Lohsiriwat S, Akaraviputh T, Boonnuch W, Methasade A, Lohsiriwat D. Hypoalbuminemia is a predictor of delayed postoperative bowel function and poor surgical outcomes in right-sided colon cancer patients. Asia Pac J Clin Nutr. 2007;16(2):213-217.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.