| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 1, February 2025, pages 59-69
Discordant Responses Between Imaging Examination and Surgical Pathology of Head and Heck Squamous Cell Carcinoma After Neoadjuvant Immunotherapy Combined With Chemotherapy
Yu Dong Ninga, b, Yi Xuan Songa, b, Yu Qin Hea, Han Lia, Shao Yan Liua, c
aDepartment of Head and Neck Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
bThese authors contributed equally to this study.
cCorresponding Author: Shao Yan Liu, Department of Head and Neck Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100124, China
Manuscript submitted September 18, 2024, accepted November 21, 2024, published online December 31, 2024
Short title: Imaging-Pathology Discordance in HNSCC After Therapy
doi: https://doi.org/10.14740/wjon1973
| Abstract | ▴Top |
Background: We here investigated the value of imaging examination in evaluating tumor remission-based surgery in patients with head and neck squamous cell carcinoma (HNSCC), who had undergone neoadjuvant immunotherapy combined with chemotherapy (NICC).
Methods: HNSCC patients who underwent NICC and surgery from May 2021 to September 2023 were retrospectively analyzed. All patients had to undergo imaging examination evaluation, including enhanced computed tomography (CT) and enhanced magnetic resonance (MR) imaging before and after NICC. Data related to clinical parameters, complete response of the primary site (PrCR), complete response of the primary site and the lymph node (PLCR), complete response of the lymph node (LCR), and tumor response (TR), were gathered. The paired Chi-square test and t-test were conducted to analyze the differences in responses between imaging examination and pathology. Binary logistic regression was applied to analyze the relevant clinical factors of differences in responses.
Results: In total, data of 41 patients were included in this study. Significant discordant responses were observed between enhanced CT, magnetic resonance imaging (MRI), and pathology in PrCR (4.9%, 7.3% vs. 41.5%), LCR (12.2%, 7.3% vs. 53.7%), PLCR (0%, 0% vs. 31.7%), and TR (severe 29.3%,17.1% vs. 25.61%) (P < 0.05). Patients with hypopharyngeal cancer (odds ratio (OR): 7.04), oral cancer (OR: 3.64), higher neutrophil to lymphocyte ratio (NLR) (OR: 2.05), and earlier T stage (OR: 0.71) exhibited a larger response difference between enhanced CT and pathology. Patients with younger age (OR: 0.79) hypopharyngeal cancer (OR: 22.81), oral cancer (OR: 2.65), higher NLR (OR: 19.47), and earlier T stage (OR: 0.29) exhibited a larger response difference between enhanced MR and pathology.
Conclusions: Discordant responses were noted between the imaging examination and surgical pathology of HNSCC after NICC. Hypopharyngeal cancer, higher NLR, and earlier T stage may predict a higher response difference.
Keywords: Pathological complete response; Neoadjuvant immunotherapy; Head neck squamous carcinoma; Pathological remission; Immune checkpoint inhibitors; Pathological tumor response; Imaging examination
| Introduction | ▴Top |
Head and neck squamous cell carcinoma (HNSCC) accounts for 90% of all head and neck malignancies [1, 2]. Most patients are diagnosed with locally advanced HNSCC (LAHNSCC) at presentation; while LAHNSCC is potentially curable, the 5-year survival rate for high-risk patients is < 50% [3]. Immunotherapy with immune checkpoint inhibitors (ICIs), such as programmed cell death protein-1 (PD-1) inhibitors (i.e., pembrolizumab, nabumab), has become the new therapeutic standard for patients with recurrent/metastatic HNSCC. In the KEYMAT-048 clinical trial, pembrolizumab alone or in combination with chemotherapy significantly improved overall survival (OS) and was approved as the first-line treatment in patients with recurrent/metastatic HNSCC [4, 5]. Neoadjuvant therapy offers numerous potential therapeutic advantages for LAHNSCC patients [6]. When implemented, neoadjuvant therapy may lead to a reduction in tumor staging, thereby promoting the degradation of adjuvant therapy. In addition, neoadjuvant therapy allows early control or prevention of micrometastasis for systemic therapy. Moreover, a series of basic experiments have confirmed that ICIs can adjust immune cells and states and exert good immunotherapeutic effects [7-10]. Therefore, neoadjuvant immunotherapy seems to exert a better immunotherapeutic effect [11]. Numerous clinical trials have recently investigated neoadjuvant immunotherapy for HNSCC, and many trials have exhibited favorable effects against tumors, especially with high pathological remission rates [12-14]. Moreover, for neoadjuvant ICIs, combining a single agent with chemotherapy is more effective than monotherapy [15]. Additionally, some researchers have proposed that, instead of OS, the degree of pathological remission should be used as an outcome evaluation index of neoadjuvant immunotherapy [16, 17]. Therefore, accurate assessment of response to neoadjuvant therapy is crucial for making appropriate treatment decisions [18]. Historically, response assessment has been based on changes in tumor load, both in clinical practice and radiology [7, 19, 20]. Imaging evaluation criteria have currently been established for the solid tumor response (TR), including response evaluation criteria in solid tumors (RECIST), immune RECIST (iRECIST), and modified RECIST. However, the predictive value of these systems is unclear because of tumor inflammation and changes in interstitial or fibrotic components following immunotherapy. Few studies have investigated the effect of imaging examination in evaluating neoadjuvant immunotherapy for HNSCC patients. The present study, based on data from our center, explored the value of imaging examination in evaluating tumor remission in HNSCC patients who had received neoadjuvant immunotherapy combined with chemotherapy (NICC), aiming to better guide clinical work.
| Materials and Methods | ▴Top |
Characteristics of the cohort
A cohort of 41 patients with LAHNSCC who received NICC, followed by surgery, in the Head and Neck Surgery Department, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, from May 2021 to September 2023, were retrospectively analyzed. Patient data on gender, age, tumor type, differentiation, multiple cancers, T staging, N staging, immunotherapy cycle, neutrophil to lymphocyte ratio (NLR), and TR were collected.TR included mild, moderate, and severe TR and complete response (CR). CR encompassed complete response of the primary site (PrCR), complete response of the primary site and the lymph node (PLCR), and complete response of the lymph node (LCR). TR was evaluated through imaging examination and pathological examination. T and N staging were performed on the basis of the eighth edition of staging of the American Joint Committee on Cancer (AJCC). The clinical information of all patients was well documented
Inclusion and exclusion criteria
Inclusion criteria were all patients who: 1) had locally advanced head and neck squamous cell carcinoma that could be either resectable or not and underwent surgery after NICC; 2) were evaluated through imaging examinations including laryngoscopy, enhanced CT, and enhanced MR conducted before and after NICC to determine the clinical stage; and 3) had complete medical records.
Exclusion criteria were patients who: 1) had distant metastatic HNSCC; 2) could not tolerate surgery; 3) had incomplete imaging evaluation; and 4) had incomplete medical records.
CT and MR examination
All patients underwent 64-slice spiral CT scan (light speed; VCT or Discovery HD750; GE Healthcare, USA). After plain CT scan, patients received contrast media (1.2 mL/kg; Omnipaque 350 mg I/mL; GE Healthcare, USA), at a flow rate of 3.5 mL/s, then 40 mL saline solution was injected into the elbow vein using a power syringe (Medrad Stellant, Indianola, PA) at a flow rate of 3.0 mL/s. The arterial phase occurred 15 s after the injection of the contrast agent, and the venous phase occurred 45 s after the injection. To get the image, a magnetic resonance imaging (MRI) scan was performed using a 3.0T scanner (GE Discovery MR 750, GE Medical Systems) with an eight-channel head and neck phased alignment ring. Axial rapid destruction gradient echo (FSPGR) contrast-enhanced T1WI was used with a power syringe, 60 s after an intravenous dose of gadopentetate dimeglutamine (Magnevist, Bayer, Leverkusen, Germany) (0.2 mL/kg body weight, 1.5 mL/s).
Pathological evaluation
A biopsy of the primary sites was collected in all patients before NICC, and their cancer was pathologically confirmed as squamous cell carcinoma with or without the biopsy of the lymph node. After surgery, all imaging reports are were reviewed by two experienced radiologists. Pathologically, the TR was quantified based on the proportion of tumor necrosis, keratin fragments, and giant cells/histiocytes in the surgically removed tissue as mild TR (0-20%), moderate TR (20-80%), and severe TR (80-100%). Radiologically, TR was evaluated on the basis of tumor remission as mild TR (0-20%), moderate TR (20-80%), and severe TR (80-100%). By imaging examination, remission was evaluated on the basis of RECIST. CR was defined as no tumor noted during imaging examination or pathological examination. NLR were recorded before NICC from blood routine test. For blood analysis, a fully automated blood analysis device (Beckman Coulter International Trading Company, Model: LH750) is used to perform routine blood tests, along with a companion reagent, a vacuum anticoagulant tube (BD Corporation, USA, model: EDTA-K2). After fasting for 8 h, 2 mL cubitus venous blood was drawn the following day. Three clinically experienced operators participated in the testing to ensure that the testing process is error-free.
Treatment method
All the patients were subjected to standard clinical treatment. All patients received NICC, followed by surgery. NICC included the use of programmed death protein-1 (PD-1) inhibitors (200 mg), such as pabolizumab, triprilimab, tirellizumab, or sindillimab, in combination with cisplatin (75 mg/ m2) and paclitaxel (175 mg/ m2), administered every 21 days on the first day of the cycle. Surgery involved the removal of primary sites and neck dissection, without or with repair and reconstruction.
Statistical method
The pairwise paired Chi-square test and t-test was conducted to analyze differences in responses between imaging examination and pathology. The correlation between the enhanced MR remission of PrCR was analyzed through the receiver operating characteristic (ROC) curve and Chi-square test. Binary logistic regression was performed to analyze the relevant clinical factors of response differences by odds ratio (OR). All statistical analyses were performed using SPSS.27.
Ethics approval
The research program was approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences. All participants and patients agreed to participate in this research program. The study was conducted in compliance with ethical guidelines for human research.
| Results | ▴Top |
Patient characteristics
A total of 41 patients with HNSCC were included in this study (Table 1, Fig. 1). The patients were assigned to two groups according to their gender and then divided into four groups according to tumor types, namely oropharyngeal cancer (11, 26.8%), hypopharyngeal cancer (25, 61.0%), oral cancer (4, 9.8%), and laryngeal cancer (1, 2.4%). The number of patients with multiple cancers in this cohort was 4 (9.8%). These cohorts were divided into high (5, 12.2%), middle (23, 56.1%), and low differentiation (13, 31.7%) based on pathological differentiation. The patients were assigned to three groups on the basis of immunotherapy cycles, namely 1 (2, 4.9%), 2 (31, 75.6%), and 3 (7, 17.1%). They were also categorized into four groups by ICIs, namely pabolizumab (n = 27, 65.9%), tirellizumab (n = 10, 24.4%), triplimab (n = 3, 7.3%), and sindilizumab (n = 1, 2.4%). The mean age and NLR of the patients were 59.61 and 1.68 years, respectively.
 Click to view | Table 1. Characteristics of the Cohort |
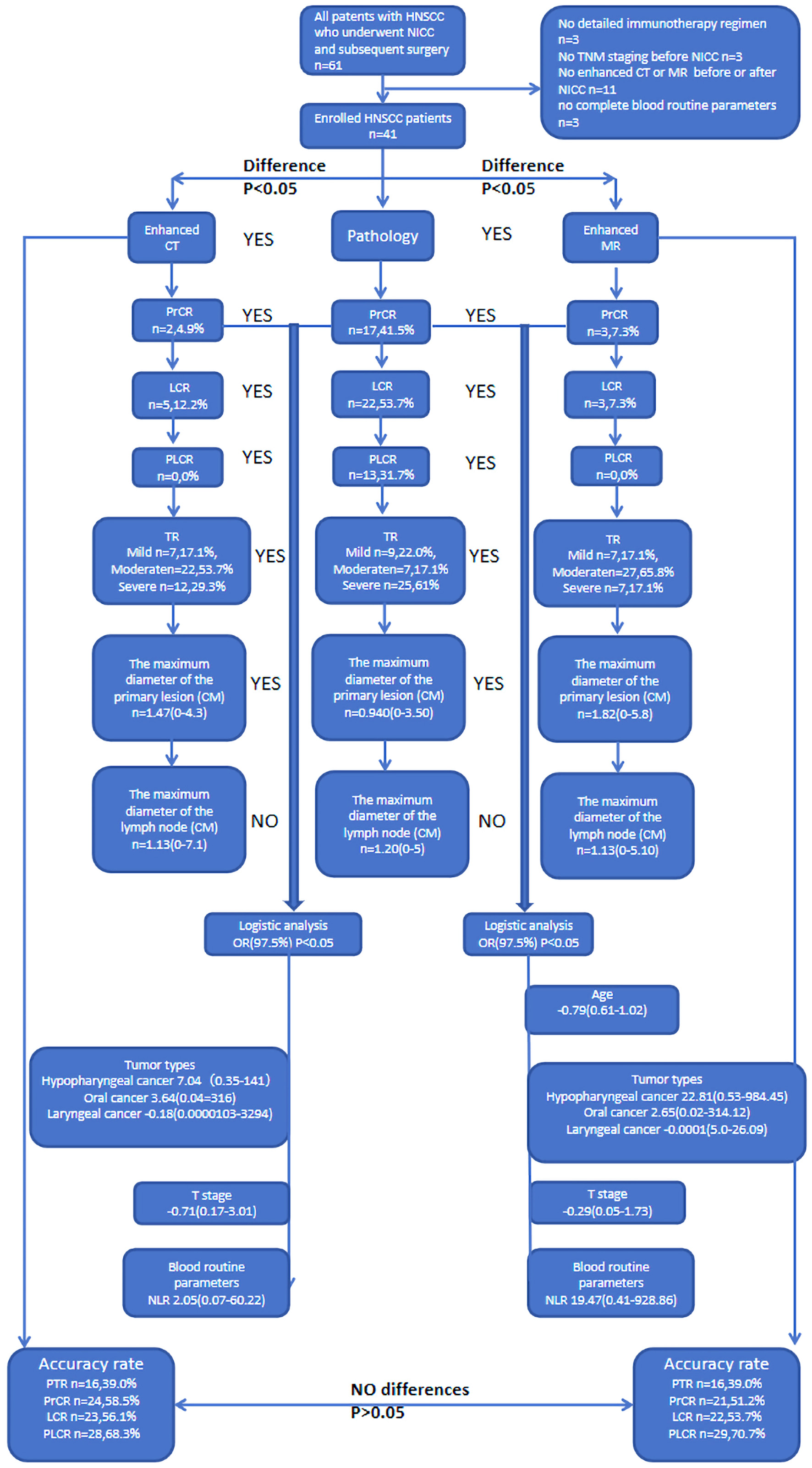 Click for large image | Figure 1. Flow chart. |
The response difference between pathological and imaging examinations on TR
Significant discordant responses were noted between enhanced CT and pathology in PrCR (4.9% vs. 41.5%), LCR (12.2% vs. 53.7%), PLCR (0% vs. 31.7%), and TR (severe 29.3% vs. 25.61%) (P < 0.05) (Table 2, Fig. 2). The maximum diameter of the primary lesion noted during pathological examination was significantly larger than that observed on enhanced CT (1.47 cm vs. 0.940 cm) (P = 0.022). However, the maximum diameter of the lymph node exhibited no significant differences between pathological examination and enhanced CT. Significant discordant responses were observed between enhanced MR and pathology in PrCR (7.3% vs. 41.5%), LCR (7.3% vs. 53.7%), PLCR (0% vs. 31.7%), and TR (severe 17.1% vs. 25.61%) (P < 0.05) (Table 3, Fig. 2). The maximum diameter of the primary lesion observed during pathological examination was significantly larger than that observed on enhanced MR (1.82 cm vs. 0.940 cm) (P = 0.022). However, the maximum diameter of the lymph node did not differ significantly between pathological examination and enhanced MR. Figure 3 shows the discordant responses between imaging examination and surgical pathology of HNSCC after NICC.
 Click to view | Table 2. The Characters and the Difference Between Enhanced CT and Pathology |
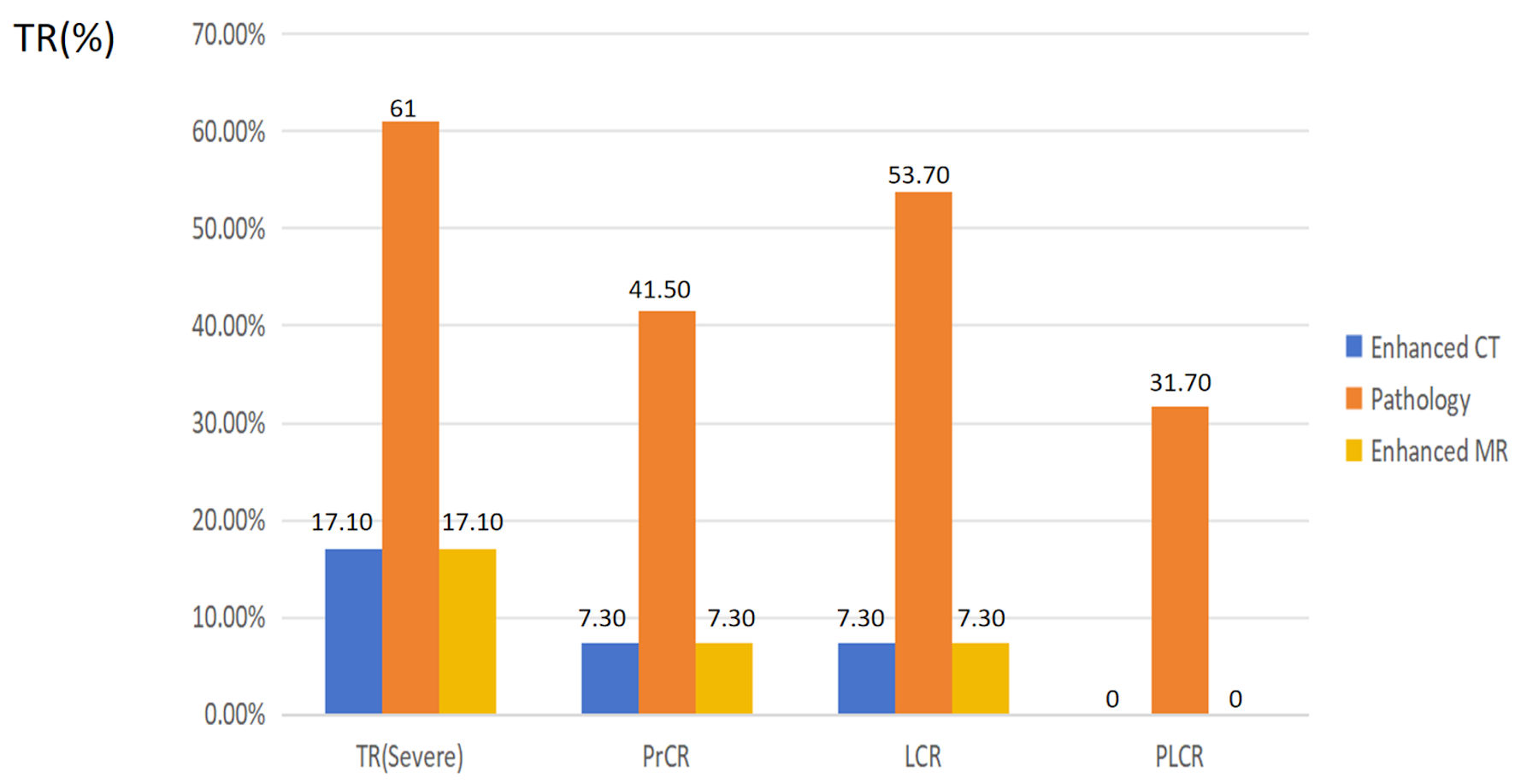 Click for large image | Figure 2. The characters and the difference between enhanced CT, MR and pathology. PrCR: complete response of the primary site; PLCR: complete response of the primary site and the lymph node; LCR: complete response of the lymph node; TR: tumor response; CT: computed tomography; MR: magnetic resonance. |
 Click to view | Table 3. The Characters and the Difference Between Enhanced MR and Pathology |
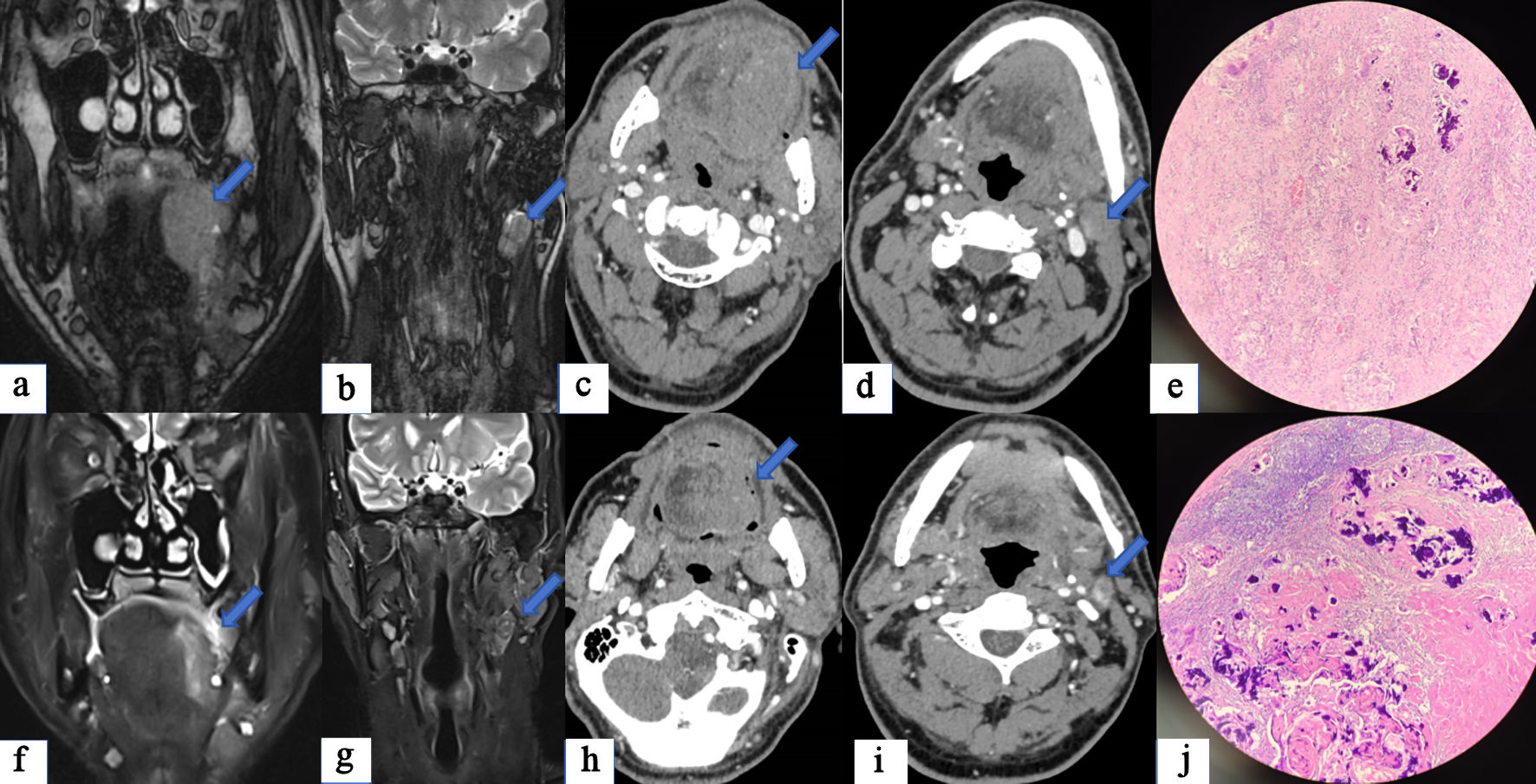 Click for large image | Figure 3. A case of tongue squamous cell carcinoma with cervical lymph node metastasis treated by surgery after two cycles of NICC. (a) Before NICC, enhanced MRI suggested that an abnormal signal mass is seen on the left side of the tongue, with irregular shape and maximum cross-sectional area of approximately 5.5 × 3.8 cm (arrow). (b) Multiple enlarged lymph nodes are seen on the left side of the neck, with the largest one having a short axis of approximately 1.7 cm (arrow). (c) Before NICC, enhanced CT showed a lesion on the left side of the tongue with an irregular shape and a maximum cross-sectional area of approximately 5.3 × 3.0 cm (arrow). (d) There are multiple lymph nodes in the neck, with the largest having a short diameter of about 1.7 cm (arrow). After NICC, enhanced MRI suggested that abnormal signal shadow is seen on the left side of the tongue, with irregular shape, smaller range and uneven signal. (f) The maximum cross-section was about 4.3 × 2.1 cm (arrow), which was considered to be better after NICC than before. (g) Most of the lymphatic nodes of the left neck were smaller than before, and the short diameter of the larger ones was about 1.4 cm (arrow). (h) After NICC, enhanced CT revealed a left-sided mass in the tongue, smaller than before, with unclear boundaries, and the current range was about 4.5 × 3.2 cm (arrow). (i) Multiple lymph nodes in the left neck were smaller than before, and the short diameter of the larger ones was about 1.3cm (arrow). The postoperative pathology report indicated PrCR (e) and LCR (j), while imagine examination still indicated the presence of tumor (f-i). NICC: neoadjuvant immunotherapy combined with chemotherapy; CT: computed tomography; MRI: magnetic resonance imaging; PrCR: complete response of the primary site; LCR: complete response of the lymph node. |
The related factor analysis to response differences
We analyze the relevant clinical factors of response differences by conducting binary logistic regression of data including gender, age, tumor types, multiple cancers, differentiation, T stage, immunotherapy cycles, ICI types, and NLR. The patients with hypopharyngeal cancer (OR: 7.04), oral cancer (OR: 3.64), higher NLR (OR: 2.05), and earlier T stage (OR: 0.71) exhibited a larger response difference between enhanced CT and pathology (Table 4). The patients with younger age (OR: 0.79), hypopharyngeal cancer (OR: 22.81), oral cancer (OR: 2.65), higher NLR (OR: 19.47), and earlier T stage (OR: 0.29) exhibited a larger response difference between enhanced MR and pathology (P < 0.05) (Table 5).
 Click to view | Table 4. The Multivariate Analysis of the Difference Between Enhanced CT and Pathology on TR |
 Click to view | Table 5. The Multivariate Analysis of the Difference Between Enhanced MR and Pathology on TR |
| Discussion | ▴Top |
The RECIST 1.0 standard was released in 2000 and was updated to version 1.1 in 2009. Although these standards simplify the measurement steps and improve the accuracy of measurements on the basis of the World Health Organization (WHO) standards, the efficacy is continued to be judged according to changes in tumor size, which is measured using traditional imaging techniques (CT, MR). In fact, many tumors, such as lymphoma, sarcoma, and liver cancer, exhibit no significant change in volume after treatment. Therefore, the RECIST criteria does not reflect the true efficacy. In 2017, the RECIST Working Group and its Immunotherapy Subcommittee published the criteria for evaluation of iRECIST. These criteria clarified how to evaluate remission and progress in immunotherapy trials [21]. However, with the advent of the immunotherapy era, many problems that did not appear during tumor evaluation before, such as false progression and new lesions, have now emerged. These new problems are a result of immunotherapy itself, and the essence of immunotherapy is an attack response of immune cells activated by the body to the tumor, which is a sign indicative of the effectiveness of tumor treatment. However, if judged according to iRECIST criteria, this will be considered tumor progression, which will have a certain impact on the patient’s next clinical treatment.
The response difference between pathology and imaging examination on TR
At present, the effect of immunotherapy cannot be accurately evaluated through imaging examination of solid tumors. Using the RECIST or iRECIST criteria, we here obtained TR rates of enhanced CT and MR, according to the maximum tumor diameter at baseline before and after NICC. The evaluation efficacy of imaging examination for HNSCC after NICC was compared with that of postoperative pathology, which was used as the gold standard. Significant discordant responses were observed between enhanced CT and MR, and pathology in PrCR (4.9%, 7.3% vs. 41.5%), LCR (12.2%, 7.3% vs. 53.7%), PLCR (0%, 0% vs. 31.7%), and TR (severe 29.3%, 17.1% vs. 25.61%) (P < 0.05). Figure 3 shows a case of tongue squamous cell carcinoma with cervical lymph node metastasis that was treated by surgery after two cycles of NICC. The postoperative pathology report indicated PrCR (Fig. 3e) and LCR (Fig. 3j), while imagine examination still indicated the presence of tumor (Fig. 3f-i). All results of the response comparison between imaging and pathological examinations were obtained through the one-to-one matching test. The results of imaging examination did not match those of pathology, which may have been because of interference by inflammatory tumor necrosis caused by immunotherapy. This conclusion was supported by our findings, which show the low rate of CR, as assessed by imaging examination, due to the presence of inflammatory substances after immunotherapy. The maximum diameter of the primary lesion observed through pathology was significantly larger than that observed on enhanced CT (1.47 cm vs. 0.940 cm) (P = 0.022). The maximum diameter of the primary lesion observed through pathology was significantly larger than that noted on enhanced MR (1.82 cm vs. 0.940 cm) (P = 0.022). This phenomenon is very interesting, but the specific mechanism remains unclear. This may have occurred due to data bias. However, the maximum diameter of the lymph node exhibited no significant differences between pathology and enhanced CT and enhanced MR (P > 0.05). This may be due to the fact that primary and lymph nodes respond differently to immunotherapy. Merlino et al observed inconsistent therapeutic effects of nivolumab between the primary tumor site and metastatic lymph nodes in patients [22]. Certainly, data bias may also play a role. Although their data support the use of early radiographic response to assess the immunotherapy treatment effect in HNSCC, their research was based on radiographic volumetric response, not RECIST criteria. We have verified this with our own data by the classical RECIST criteria and found that discordant responses were noted between the imaging examination and surgical pathology of HNSCC after NICC.
The related factor analysis to response differences
The results of radiographic examination and pathological evaluation of TR were confirmed to be inconsistent. Differentiating clinical factors are being sought to better guide clinical work. The relevant clinical factors of response differences were analyzed by conducting binary logistic regression, with data including gender, age, tumor types, multiple cancers, differentiation, T stage, immunotherapy cycles, ICI types, and NLR. The patients with hypopharyngeal cancer (OR: 7.04), oral cancer (OR: 3.64), higher NLR (OR: 2.05), and earlier T stage (OR: 0.71), exhibited a larger response difference between enhanced CT and pathology (Table 4). The patients with younger age (OR: 0.79), hypopharyngeal cancer (OR: 22.81), oral cancer (OR: 2.65), higher NLR (OR: 19.47), and earlier T stage (OR: 0.29), exhibited a larger response difference between enhanced MR and pathology (P < 0.05). Anatomic complexity may affect the TR assessment by enhanced CT and MR. In the case of hypopharyngeal cancer, the complex anatomy of the hypopharynx, along with the inflammatory interference of immunotherapy, may increase the difficulty of TR assessment by enhanced CT and MR (OR: 7.04) (Table 5). The immune response was possibly stronger in early tumors. A higher NLR suggests a higher inflammatory response, thereby increasing differentiation. It has also been reported by other study that the predictive value of RECIST may be low due to changes in the inflammatory and interstitial or fibrotic components of tumors that may affect imaging findings [23, 24]. Thus, in clinical practice, we must pay attention to the large differences between patients with imaging and pathological examinations, especially when patients with younger age, hypopharyngeal cancer, oral cancer, higher NLR, and earlier T stage.
Conventional imaging tools, such as enhanced CT and MR, have some limits to evaluate the pathology after NICC. To guide clinical practice about NICC, radiomics may be an approach to compensate for the difference in the pathological response of tumors observed on imaging examination. Immunotherapy can induce an early treatment response in some HNSCC patients. These reactions cannot be diagnosed using conventional imaging parameters. According to a study [23], MR diffusion-weighted imaging (DWI) may be able to diagnose these reactions. On analyzing imaging data from 24 patients with advanced squamous cell carcinoma before and after immunotherapy, those authors found that round tumors with a smaller diameter before treatment were more likely to respond. Lower tumor skewness and overall skewness after treatment were associated with a better treatment response than that before treatment. While additional studies with larger data sets are warranted to definitively link DWI parameters to immunotherapy outcomes, the present study may offer guidance on research directions. In fact, the emergence of radiomic signatures for predicting immunotherapy responses has caused an upsurge of research in the field. However, the lack of standardized protocols and validation poses a major challenge to its application in clinical practice. Although many radiomics studies have predicted responses across tumor types, there are still inconsistencies in data selection, model construction, and outcome definition. These radiomic-based analyses still need to be validated in larger clinical studies before they can be implemented in clinical practice.
Many studies have adopted other imaging tools, but the results are unsatisfactory. An assessment of metabolic responses based on 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) revealed that pathological responses can be detected in HNSCC patients receiving neoadjuvant immunotherapy by using a calculated δ-value of total glycoenzymolysis of the lesion. Although the technique had an accuracy of 94% at the primary tumor site, it was limited by the relatively large tumor volume required to assess the variability of the total lesion glycolysis (TLG) and FDG influx in immune cells during immunotherapy treatment, which could produce false-positive results [24, 25]. The metabolic approach (i.e., PET/CT) offers complementary information on two-dimensional tumor shrinkage, thereby clarifying that the reduction in 18F-FDG uptake is directly related to the density of live tumor cells. Additionally, while focal and asymmetrical 18F-FDG uptake in primary tumor areas or lymph nodes may indicate residual lesions, no clear standardized uptake value (SUV) boundary exists between benign and malignant lesions. In patients treated with ICIs, resulting immune infiltration may further complicate PET/CT interpretations. The study of Shah et al revealed that no correlation exists between changes in FDG uptake and pathological TR after neoadjuvant PD-1 axis suppression [26]. Vos et al [24] recently suggested that changes in the metabolic tumor volume and TLG have some merit in detecting pathological responses at the primary site. However, numerous false-positive results were reported when the response of cervical lymph nodes was assessed using metabolic methods [24]. Therefore, new imaging tools or imaging agents need to be invented or identified. The complexity of monitoring tumor response in patients treated with ICIs has prompted the development of novel radiotracers, particularly PET/CT PD-L1 tracers currently used in clinical practice, which have a strong correlation with PD-L1 status through immunohistochemical measurements [27]. In addition, these tracers are able to show heterogeneity of PD-L1 expression between different patients and within tumor lesions in the same patient on PET/CT, even more accurately than immunohistochemically stained biopsy samples [28]. However, to our knowledge, only a handful of imaging probes are currently in the clinical research stage and have not yet been approved by the Food and Drug Administration for clinical use [29]. So, more research is needed in the future.
However, our study also had some limitations. The overall cohort was small. Moreover, the study had a predominance of patients with oropharyngeal and hypopharyngeal cancers, and those with oral and laryngeal cancers were limited, which may have introduced a potential bias. The study findings must be validated in large sample sizes or multi-center studies. Further, the heterogeneity of different immunological agents used may have introduced variability in the results. More studies, especially prospective studies, are needed in the future to remedy these shortcomings.
Conclusions
Discordant responses exist between imaging examination and pathology of HNSCC after NICC. Hypopharyngeal cancer, higher NLR, and earlier T stage may help predict a higher response difference. Other imaging examination tools or radiomics methods should be explored in future studies.
Acknowledgments
None to declare.
Financial Disclosure
The study was funded by Beijing Hope Run Special Fund of Cancer Foundation of China (Grant No. LC2017L04).
Conflict of Interest
The authors declare that they have no conflict of interest to report regarding the present study.
Informed Consent
Patient consent for publication was obtained for the study.
Author Contributions
Study conception and design: Yu Dong Ning and Shao Yan Liu. Data collection: Yu Qin He. Analysis and interpretation of results: Yi Xuan Song and Han Li. Draft manuscript preparation: Yu Dong Ning and Yi Xuan Song. All authors reviewed the results and approved the final version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386-396.
doi pubmed - Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33.
doi pubmed - Brockstein B, Haraf DJ, Rademaker AW, Kies MS, Stenson KM, Rosen F, Mittal BB, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol. 2004;15(8):1179-1186.
doi pubmed - Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr., Psyrri A, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928.
doi pubmed - Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, Le QT, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7(1):184.
doi pubmed - Hanna GJ, Adkins DR, Zolkind P, Uppaluri R. Rationale for neoadjuvant immunotherapy in head and neck squamous cell carcinoma. Oral Oncol. 2017;73:65-69.
doi pubmed - Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, Zahurak M, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976-1986.
doi pubmed - Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355(6332):1423-1427.
doi pubmed - Memarnejadian A, Meilleur CE, Shaler CR, Khazaie K, Bennink JR, Schell TD, Haeryfar SMM. PD-1 blockade promotes epitope spreading in anticancer CD8(+) T cell responses by preventing fratricidal death of subdominant clones to relieve immunodomination. J Immunol. 2017;199(9):3348-3359.
doi pubmed - Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, McNamara KL, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med. 2019;25(8):1251-1259.
doi pubmed - Friedman J, Moore EC, Zolkind P, Robbins Y, Clavijo PE, Sun L, Greene S, et al. Neoadjuvant PD-1 immune checkpoint blockade reverses functional immunodominance among tumor antigen-specific T cells. Clin Cancer Res. 2020;26(3):679-689.
doi pubmed - Leidner R, Crittenden M, Young K, Xiao H, Wu Y, Couey MA, Patel AA, et al. Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma. J Immunother Cancer. 2021;9(5):e002485.
doi pubmed - Ferris RL, Spanos WC, Leidner R, Goncalves A, Martens UM, Kyi C, Sharfman W, et al. Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial. J Immunother Cancer. 2021;9(6):e002568.
doi pubmed - Vos JL, Elbers JBW, Krijgsman O, Traets JJH, Qiao X, van der Leun AM, Lubeck Y, et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat Commun. 2021;12(1):7348.
doi pubmed - Yilmaz E, Ismaila N, Bauman JE, Dabney R, Gan G, Jordan R, Kaufman M, et al. Immunotherapy and biomarker testing in recurrent and metastatic head and neck cancers: ASCO guideline. J Clin Oncol. 2023;41(5):1132-1146.
doi pubmed - Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24:1649-1654.
- Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, Krijgsman O, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655-1661.
doi pubmed - Xu Y, Ma D, Qin Y, Li S, Li J, Jiang Y, Wang M, et al. Is response evaluation criteria in solid tumors (RECIST) effective in patient selection for radical resection after neoadjuvant immunotherapy with advanced NSCLC? Thorac Cancer. 2023;14(17):1635-1639.
doi pubmed - Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
doi pubmed - William WN, Jr., Pataer A, Kalhor N, Correa AM, Rice DC, Wistuba, II, Heymach J, et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2013;8(2):222-228.
doi pubmed - Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143-e152.
doi pubmed - Merlino DJ, Johnson JM, Tuluc M, Gargano S, Stapp R, Harshyne L, Jr., Leiby BE, et al. Discordant responses between primary head and neck tumors and nodal metastases treated with neoadjuvant nivolumab: correlation of radiographic and pathologic treatment effect. Front Oncol. 2020;10:566315.
doi pubmed - van der Hulst HJ, Vos JL, Tissier R, Smit LA, Martens RM, Beets-Tan RGH, van den Brekel MWM, et al. Quantitative diffusion-weighted imaging analyses to predict response to neoadjuvant immunotherapy in patients with locally advanced head and neck carcinoma. Cancers (Basel). 2022;14(24):6235.
doi pubmed - Vos JL, Zuur CL, Smit LA, de Boer JP, Al-Mamgani A, van den Brekel MWM, Haanen J, et al. [(18)F]FDG-PET accurately identifies pathological response early upon neoadjuvant immune checkpoint blockade in head and neck squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2022;49(6):2010-2022.
doi pubmed - Driessen JP, van Kempen PM, van der Heijden GJ, Philippens ME, Pameijer FA, Stegeman I, Terhaard CH, et al. Diffusion-weighted imaging in head and neck squamous cell carcinomas: a systematic review. Head Neck. 2015;37(3):440-448.
doi pubmed - Shah H, Wang Y, Cheng SC, Gunasti L, Chen YH, Lako A, Guenette J, et al. Use of Fluoro-[18F]-Deoxy-2-D-Glucose positron emission tomography/computed tomography to predict immunotherapy treatment response in patients with squamous cell oral cavity cancers. JAMA Otolaryngol Head Neck Surg. 2022;148(3):268-276.
doi pubmed - Niemeijer AL, Hoekstra OS, Smit EF, de Langen AJ. Imaging responses to immunotherapy with novel PET tracers. J Nucl Med. 2020;61(5):641-642.
doi pubmed - Niemeijer AN, Leung D, Huisman MC, Bahce I, Hoekstra OS, van Dongen G, Boellaard R, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun. 2018;9(1):4664.
doi pubmed - McCarthy CE, White JM, Viola NT, Gibson HM. In vivo imaging technologies to monitor the immune system. Front Immunol. 2020;11:1067.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.