| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 1, February 2025, pages 51-58
Prevalence and Clinical Outcomes of Human Epidermal Growth Factor Receptor 2 Expression in Patients With Advanced Urothelial Carcinoma
Ivan A. Ortiz-Calderona, Luis Felipe Arias-Ruizb, Rita Dorantes-Herediab, Jose Manuel Ruiz-Moralesa, c
aOncology Center, Hospital Medica Sur, Mexico City, Mexico
bAnatomical Pathology Department, Hospital Medica Sur, Mexico City, Mexico
cCorresponding Author: Jose Manuel Ruiz-Morales, Oncology Center, Hospital Medica Sur, Mexico City, Mexico
Manuscript submitted September 11, 2024, accepted November 27, 2024, published online December 31, 2024
Short title: Outcomes of HER2 in Urothelial Cancer
doi: https://doi.org/10.14740/wjon1966
| Abstract | ▴Top |
Background: The prognosis for urothelial carcinoma remains poor, with limited therapeutic options, emphasizing the need for further research into targeted therapies. The prognostic and predictive significance of human epidermal growth factor receptor 2 (HER2) expression in urothelial carcinoma remains unclear, with previous studies reporting conflicting results.
Methods: We conducted a retrospective analysis of advanced urothelial carcinoma cases diagnosed between January 2017 and December 2022. HER2 status was prospectively determined using the Leica CB11 antibody on available biopsy specimens. Patient data, tumor characteristics, and survival outcomes were retrieved from hospital records for analysis.
Results: Of the 84 patients initially identified with muscle-invasive disease, HER2 immunohistochemistry (IHC) was performed on 50 samples. Among these, 54% exhibited HER2 scores ≥ 1+, with 22% classified as HER2-positive (3+ score by IHC), 10% as equivocal (2+ score by IHC), and 22% as HER2-low (1+ score by IHC). The distribution of HER2 score ≥ 1+ tumors included 25.7% in the bladder, 20.0% in the renal pelvis, and none in the ureter. HER2-positive (3+ score by IHC) tumors were all histological grade 3. Among these patients, 13.4% presented with localized disease, 20% with locally advanced disease, and 50% with metastatic disease at the time of diagnosis. Notably, 42.8% of recurrent tumors originating from the renal pelvis and 62.5% of those from the bladder exhibited HER2 scores ≥ 1+. Among patients diagnosed with non-metastatic disease, 100% with renal pelvis tumors and 75% with bladder tumors experienced metastatic recurrence if they were HER2-positive (3+ score by IHC). The overall survival for HER2-negative patients was 31.0 months (95% confidence interval (CI): 15.29 - 66.70) compared to 13.0 months (95% CI: 7.32 - 18.68) in the HER2 score ≥ 1+ population (P = 0.0029).
Conclusions: In this cohort of Mexican patients with urothelial carcinoma, HER2 expression was observed in 54.4% of cases. HER2-positive (+3 by IHC) tumors were associated with higher histological grade and worse prognostic outcomes, including increased recurrence, progression, and mortality.
Keywords: Urothelial carcinoma; HER2 expression; Renal pelvis tumors; Prognostic biomarkers; Immunohistochemistry; Muscle-invasive bladder cancer; Metastatic recurrence; Tumor grade
| Introduction | ▴Top |
Despite recent advances in understanding the biology of urothelial carcinoma and the introduction of novel treatments, the prognosis for this disease remains poor, with therapeutic options severely limited [1]. This highlights the urgent need for research into more effective drugs, particularly those targeting specific receptors [2].
One of the most extensively studied receptors in oncology is the human epidermal growth factor receptor 2 (HER2), encoded by the ERBB2 gene on chromosome 17. HER2, a member of the epidermal growth factor receptor (EGFR) family, plays a pivotal role in activating multiple signaling pathways, including MAPK, PI3K-PKB/AKT, and PKC, all of which are crucial for regulating cell growth, differentiation, and survival [3].
HER2-targeted therapies have revolutionized the management and prognosis of breast cancer, and they have also demonstrated remarkable efficacy in the treatment of gastric adenocarcinoma, establishing HER2 blockade as a standard therapy for tumors expressing the HER2 receptor. Additionally, antibody-based HER2 receptor blockade has shown statistically significant, albeit modest, improvements in the outcomes of patients with endometrial carcinoma, colon adenocarcinoma, and salivary gland tumors. More recently, it has emerged as a viable later-line treatment option for lung cancer [4].
Currently, the prognostic significance of HER2 expression in urothelial carcinoma remains uncertain, with previous studies producing mixed results. Despite this lack of clarity, HER2-targeted therapies such as trastuzumab, trastuzumab emtansine (TDM1), apatinib, lapatinib, and neratinib have shown limited efficacy in the management of urothelial carcinoma [5-7]. Moreover, there are no standardized criteria for HER2 testing in urothelial carcinoma, and unlike breast cancer, there are no established immunohistochemistry (IHC) tests or validated antibody clones specific to this tumor type. This absence of standardized testing contributes to variability in the reported findings [8]. A systematic literature review by Scherrer et al [9] revealed that the prevalence of HER2 expression in urothelial carcinoma ranged from 6.7% to 37.5% across multiple studies, with a weighted average of 13.0% in cases of locally advanced or metastatic disease. To address these gaps, this study aims to evaluate the prevalence of HER2 expression and its clinical implications in a cohort of Mexican patients with urothelial carcinoma. Additionally, it seeks to correlate HER2 status with demographic, clinical, and pathological characteristics, as well as patient outcomes.
| Materials and Methods | ▴Top |
Within the Pathology Department database at our hospital, we identified all patients diagnosed with urothelial carcinoma confirmed by histological evaluation between January 2017 and December 2022. Our study included individuals aged 18 years or older with muscle-invasive urothelial carcinoma. The dataset collected included detailed information on age at diagnosis, family history, tobacco and alcohol use, comorbid conditions (specifically diabetes and hypertension), primary tumor location, tumor grade, histological growth pattern, depth of invasion, clinical stage at diagnosis, metastatic sites (either at diagnosis or during recurrence), initial treatment administered, treatment response, disease-free survival, progression-free survival, and overall survival.
To maintain specificity, we excluded patients with non-muscle invasive disease, those with non-urothelial bladder cancers, and individuals whose tissue samples were unavailable for IHC testing or had incomplete clinical information in the hospital records.
HER2 status was determined using the Leica CB11 antibody (NCL-L-CB11 Leica Biosystems Newcastle) at a 1:250 dilution. The results were classified as positive (score 3+), equivocal (score 2+), low (score 1+), or negative (score 0) according to the criteria outlined in the “HER2 Testing in Breast Cancer - 2023 Guideline Update” from the College of American Pathologists [10].
Ethical considerations
This research protocol was approved by the Hospital Ethics Committee and was conducted in strict accordance with the Mexican General Law for Healthcare Investigations, Chapter 1, Article 17, Section 1, “Health Investigation Without Harm”. No additional tissue samples were obtained specifically for this study. All required tests were prospectively performed on existing biopsy tissues stored in the Pathology Department repository. Clinical data were collected from hospital health records, ensuring the privacy and confidentiality of patient information were maintained throughout the study.
Statistical analysis
Continuous variables were summarized using either the standard deviation or the median with interquartile ranges (25th and 75th percentiles) based on the data distribution, as determined by the Shapiro-Wilk test. Inferential comparisons between continuous variables were conducted using either Student’s t-test or the Mann-Whitney U test, as appropriate. For categorical variables, frequencies and proportions were calculated, and intergroup comparisons were performed using the Chi-square test. Median overall survival, with a 95% confidence interval (CI) in months, was estimated using the Kaplan-Meier method, with continuous variables dichotomized for analysis. Comparisons of median progression-free survival and overall survival were assessed using the log-rank test. Hazard ratios (HRs) and corresponding 95% CIs were calculated using a Cox proportional hazards model. A P-value of ≤ 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 29.0.01.
| Results | ▴Top |
Between January 2017 and December 2022, we identified 84 patients in the archives of the Pathology Department with a diagnosis of advanced urothelial carcinoma, defined as at least muscle-invasive disease. Of these, 34 patients were excluded: two were reclassified as squamous cell carcinoma upon histological review, and 32 had insufficient material for HER2 IHC testing. A total of 50 patients had adequate material for HER2 testing using the Leica CB11 antibody (NCL-L-CB11 Leica Biosystems Newcastle). Of these, 39 patients had complete clinical data available for statistical analysis of clinical outcomes.
The demographic characteristics of the patients are summarized in Table 1. Among the study population, 74% were male and 26% were female, yielding a male-to-female ratio of 2.8:1. The median age at diagnosis was 79 years (range: 54 - 89). Approximately 50% of patients reported a family history of cancer, most commonly lung cancer, followed by gastric cancer, although no patients reported a family history of urothelial carcinoma. Half of the patients reported regular alcohol consumption, with 16% consuming more than four standard drinks per week. Additionally, 68% of the patients had a history of smoking, with a median of 30.7 pack-years (range: 1 - 120 pack-years). Only one patient (2%) reported recognized occupational exposure, specifically in car painting.
 Click to view | Table 1. Patient and Tumor Characteristics |
The most reported comorbidities were diabetes mellitus (34%) and hypertension (42%), with a median duration of 14.2 years for each condition. Furthermore, 22% of patients were diagnosed with a second primary cancer, either synchronously or metachronously, and one patient (2%) was diagnosed with a third primary cancer.
The primary tumor locations at diagnosis were as follows: 70% (n = 35) in the bladder, 26% (n = 13) in the renal pelvis, and 4% (n = 2) in the ureter. The most prevalent histological variant was papillary, observed in 70% (n = 35) of cases, with squamous cell differentiation seen in 16% (n = 13) of tumors. Additionally, 88% (n = 44) of tumors were classified as grade 3, and 18% (n = 9) had coexisting carcinoma in situ.
At diagnosis, disease staging revealed that 44% (n = 22) of patients presented with localized disease, 30% (n = 15) with locally advanced disease, and 12% (n = 6) with metastatic disease. Clinical stage data were unavailable for 14% (n = 7) of patients. Among patients with metastatic recurrence or metastatic disease at diagnosis, the median number of metastatic sites was 2.6 (range: 1 - 5). The most common sites of metastasis were the lung (52.6%, n = 10), liver (52.6%, n = 10), non-regional lymph nodes (42.1%, n = 8), bone (42.1%, n = 8), soft tissue (31.5%, n = 6), peritoneum (21.05%, n = 4), and bone marrow (5.2%, n = 1).
HER2 expression
HER2 status was determined using IHC in 50 patients. Among these, 54% (n = 27) had a HER2 score ≥ 1+, with 22% (n = 11) classified as HER2-positive (score 3+), 10% (n = 5) as equivocal (score 2+), and 22% (n = 11) as HER2-low (score 1+). The remaining 46% (n = 23) had a score of 0, indicating no HER2 expression.
Patient characteristics and HER2 expression
In patients with a HER2 score ≥ 1+, the median age at diagnosis was 68.6 years (range: 59 - 81 years). This group included 21.6% of males and 23.1% of females. Among patients with a family history of non-urothelial cancer, 20.8% had a HER2 score ≥ 1+, while 23.1% of patients with no familial history exhibited a HER2 score ≥ 1+. Among regular alcohol consumers, 16% had a HER2 score ≥ 1+, compared to 28.0% among non-drinkers. HER2 expression was seen in 11.8% of smokers and 43.8% of non-smokers.
Tumor characteristics and HER2 expression
HER2 scores ≥ 1+ were observed in 25.7% (n = 9) of bladder tumors, 20.0% (n = 3) of renal pelvis tumors, and 0% (n = 0) of ureteral tumors. Among grade 3 tumors, 25% (n = 11) exhibited HER2 expression, while no grade 1 tumors (n = 0) showed HER2 expression.
Of the nine patients with invasive tumors coexisting with carcinoma in situ, 44.4% (n = 4) had HER2 expression. HER2 positivity (score 3+) was observed in 19.4% (n = 7) of papillary tumors, 100% (n = 2) of micropapillary tumors, and 0% (n = 0) of solid or sarcomatoid variant tumors. HER2 positivity (score 3+) was identified in 13.4% (n = 4) of patients with localized disease, 20% (n = 3) with locally advanced disease, and 50% (n = 3) with metastatic disease.
Clinical outcomes
Among patients diagnosed with localized or locally advanced disease, 36% (n = 13) experienced metastatic recurrence. This included 27.5% (n = 8) of patients with bladder tumors and 53.8% (n = 7) with renal pelvis tumors, while none of the patients with ureter tumors experienced recurrence. Among six patients with renal pelvis tumors and HER2 scores ≥ 1+ who had non-metastatic disease at diagnosis, 50% (n = 3) experienced systemic recurrence. Of these recurrent renal pelvis tumors, 66.6% (n = 2) were HER2-positive (score 3+), while 33.3% (n = 1) were equivocal (score 2+). HER2 expression was identified in 42.8% of all recurrent tumors originating from this site. In patients with bladder tumors and HER2 scores ≥ 1+, 40% (n = 6) experienced systemic recurrence. Among recurrent bladder tumors, 60% (n = 4) were HER2-positive (score 3+), 20% were equivocal (score 2+), and 20% were HER2-low (score 1+). HER2 positivity was associated with 62.5% of all recurrent bladder tumors.
Survival analysis
The median overall survival for the entire cohort was 24 months (range: 2.3 - 45.6 months). Among HER2-positive patients (3+ score by IHC), the median overall survival was 9.0 months (95% CI: 6.08 - 11.92), compared to 31.0 months (95% CI: 10.73 - 51.26) for HER2-negative, HER2-low, and HER2-equivocal patients (scores 0, 1+, and 2+, respectively) (P = 0.13). For HER2-negative patients, the median overall survival was 31.0 months (range: 15.29 - 66.70), whereas it was 13.0 months (range: 7.32 - 18.68) in the HER2 ≥ 1+ population (P = 0.029) (Fig. 1). In the subgroup analysis, the median overall survival was 41.0 months (95% CI: 15.2 - 66.7) for HER2-negative patients, 15.0 months (range: 11.01 - 18.98) for HER2-low (score 1+), 12.0 months (range: 0.0 - 28.0) for HER2-equivocal (score 2+), and 9.0 months (range: 6.07 - 11.92) for HER2-positive (score 3+) patients (P = 1.174) (Fig. 2).
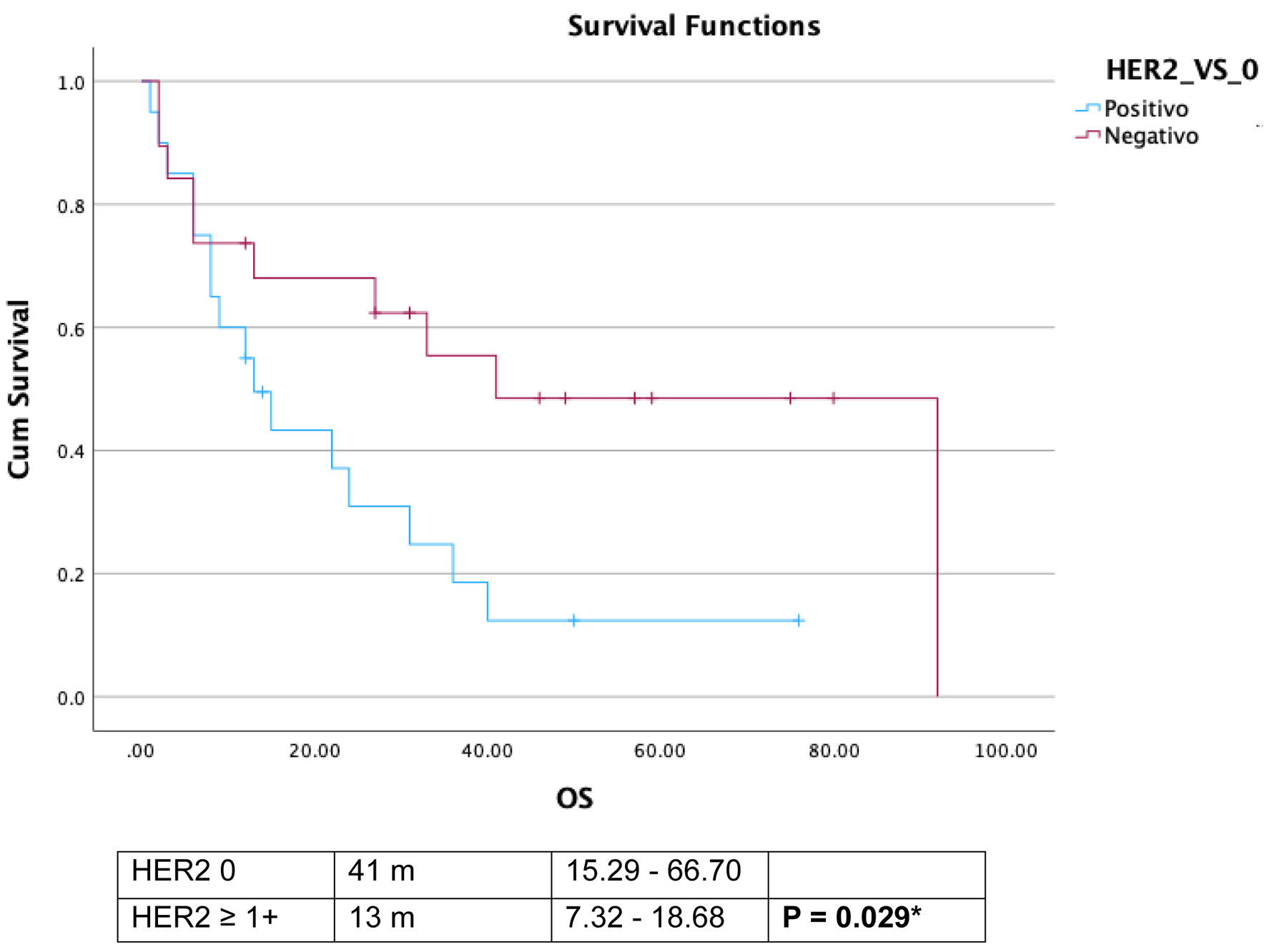 Click for large image | Figure 1. Comparative overall survival (OS) in human epidermal growth factor receptor 2 (HER2) negative and HER2 score ≥ 1+. |
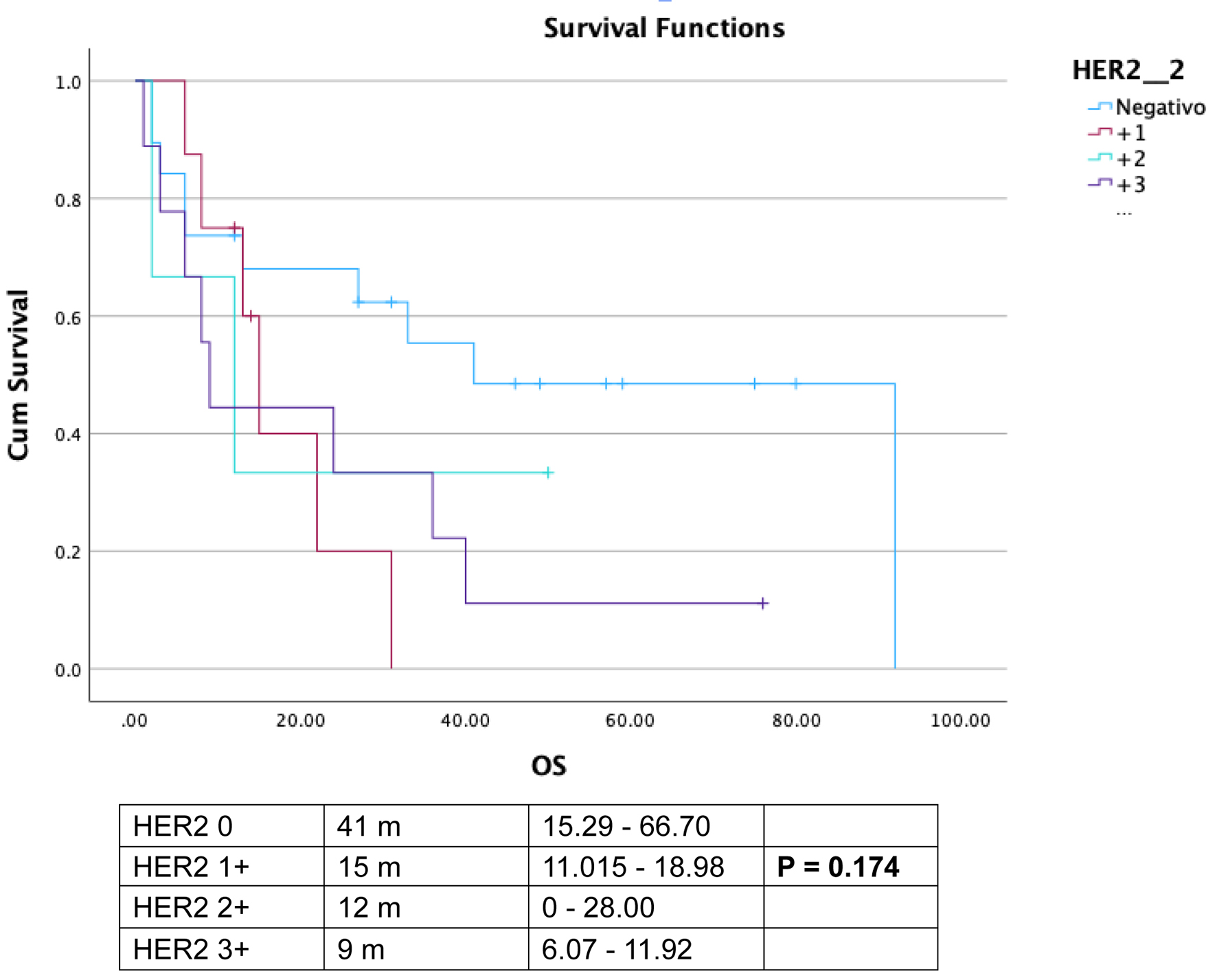 Click for large image | Figure 2. Overall survival (OS) comparison among diverse human epidermal growth factor receptor 2 (HER2) scores. |
| Discussion | ▴Top |
Globally, the understanding of the prognostic implications of HER2 expression in urothelial carcinoma continues to evolve [11]. To the best of our knowledge, this study represents the first comprehensive report of HER2 expression prevalence and clinical outcomes in a cohort of Mexican and Latin American patients, correlating these findings with demographic factors (such as sex, family history of cancer, smoking, and alcohol use) and tumor characteristics (histological grade, variant, primary localization, and stage at diagnosis). Our cohort displays the typical characteristics associated with urothelial carcinoma: older age, heavy smoking, bladder as the most common primary site, and the expected natural history of the disease [12].
HER2 expression rates in urothelial carcinoma vary widely depending on geographic population, clinical stage, and testing criteria, as well as the specific antibodies used for testing [13]. In a 2008 study by Kolla et al involving 90 Indian patients with metastatic urothelial carcinoma, the BioGenex CB11 antibody detected HER2 scores ≥ 1+ in 55.6% of cases [11]. In contrast, Necchi et al reported a prevalence of 46.2% using the Envision FLEX antibody in an Italian cohort [14]. More recently, Grigg et al found a prevalence of only 10.6% using the Ventana PATHWAY antibody in an American cohort restricted to metastatic cases [15]. It is important to note that discrepancies in HER2 expression reports often arise from the use of non-validated antibodies and inconsistent criteria for HER2 positivity. Standardized testing protocols for HER2 in urothelial carcinoma, such as those in place for breast cancer, have yet to be established [13]. In our cohort, HER2 expression (scores ≥ 1+) was observed in 54% of cases, with 22% classified as HER2-positive (score 3+), and 10% of cases deemed equivocal (score 2+). Due to the limited resources of our study, fluorescence in situ hybridization (FISH) testing was not performed to further stratify equivocal cases into HER2-low or HER2-positive categories.
HER2 plays a pivotal role in activating signaling pathways such as MAPK, PI3K/AKT, and PKC, which regulate critical processes like cell growth and survival. The overexpression of HER2 may drive aggressive behavior in urothelial carcinoma, as seen in other cancers such as breast and gastric cancer. Interestingly, HER2 expression appears more prevalent in advanced stages of disease, potentially reflecting its role in tumor progression and metastasis [15].
In our study, HER2 expression did not differ significantly by sex, family history of non-urothelial cancers, or alcohol consumption. However, non-smokers exhibited a higher prevalence of HER2 expression (43.8%) compared to smokers (11.8%). We also evaluated HER2 expression based on the primary tumor site, with 26.5% of bladder tumors and 15.4% of upper tract tumors exhibiting HER2 expression. This differs from the findings of Li et al, who reported a higher prevalence (68.85%) in upper tract urothelial carcinoma [16]. Histological variant analysis revealed that HER2 expression was highest in micropapillary tumors, with 100% showing HER2 positivity. In contrast, no HER2 expression was observed in the solid or sarcomatoid variants, though the small sample size for these subtypes limits the ability to draw definitive conclusions.
HER2 expression was more prevalent in advanced disease stages in our cohort, with rates of 13.6% in localized disease, 20% in locally advanced disease, and 50% in metastatic disease. This contrasts with findings by Scherrer et al, who reported higher HER2 expression in localized disease (60%) and lower rates in advanced disease (13%) [9].
In terms of overall survival, patients with HER2 scores ≥ 1+ had significantly shorter survival compared to HER2-negative patients. The median overall survival for HER2 ≥ 1+ patients was 13 months, compared to 41 months for HER2-negative patients (P = 0.029). However, these survival comparisons did not account for differences in disease stage or treatment. HER2-positive (score 3+) patients had a median overall survival of 9 months compared to 31 months for the HER2-negative, low and equivocal group (scores 0, 1+, 2+), though this difference did not reach statistical significance (P = 0.133). This aligns with prior studies, which have reported heterogeneity in HER2 expression between primary tumors and metastatic lesions in urothelial carcinoma [15].
Our study focused on HER2 testing at a single time point, offering a snapshot of HER2 status in the disease course. It remains unclear whether HER2 expression in metastatic disease represents early-stage HER2 positivity or an acquired resistance mechanism, as seen in breast cancer [17]. This dynamic nature of HER2 expression warrants further study. Notably, one patient in our cohort underwent both IHC and next-generation sequencing (NGS). This patient exhibited HER2 positivity (score 3+) alongside ErbB2 mutations (S310F and D769Y), as well as mutations in TP53, TERT, and BRAF, highlighting the complex genetic landscape of urothelial carcinoma. While ErbB2 amplification is common in breast cancer, this case suggests that distinct mechanisms may drive HER2 alterations in urothelial carcinoma [18].
Future studies should focus on the dynamic nature of HER2 expression throughout disease progression. Longitudinal studies assessing HER2 status from localized disease to metastatic stages may reveal whether HER2 overexpression arises as an adaptive mechanism or a primary driver. Additionally, the evaluation of antibody-drug conjugates (ADCs), such as trastuzumab deruxtecan, in HER2-expressing urothelial carcinoma could provide novel therapeutic options. Trastuzumab deruxtecan, has demonstrated activity in HER2-positive, HER2-low, and even HER2-ultralow and HER2-negative tumors [19-21]. The ongoing phase II Destiny-PanTumor02 trial, which includes patients with HER2-positive urothelial carcinoma, has shown an overall response rate of 56.3% [22, 23]. These findings highlight the potential of ADCs to reshape treatment paradigms for HER2-expressing urothelial carcinoma.
Limitations
Although our sample size is comparable to other series reported globally, certain subgroups within the cohort had limited patient numbers, making direct comparisons and conclusive findings challenging in these specific groups. This study is also limited by its single-center design, which may affect the generalizability of the results. Additionally, due to the investigator-funded nature of the research, equivocal HER2 results were not further analyzed using FISH, which may have impacted the classification of some cases. Future efforts will focus on expanding the patient cohort and conducting further analyses to provide updated and more comprehensive insights into HER2 expression in urothelial carcinoma.
Conclusions
In this cohort of Mexican patients from a single center, the prevalence of HER2 expression in urothelial carcinoma was 54.4%. Tumors expressing HER2 were associated with poorer prognostic outcomes in terms of recurrence, progression, and mortality, across all primary tumor locations within the urinary tract. While HER2 expression has yet to be definitively established as a predictive biomarker, the development of novel HER2-targeted therapies offers promising potential to reshape the treatment landscape for these patients soon.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
JMRM has provided advisory and speaker services to Ipsen, Bristol-Myers Squibb, Merck Sharp & Dohme. RDH has provided advisory and speaker services to Merck Sharp & Dohme, AstraZeneca. The rest of authors declare no conflict of interest.
Informed Consent
Not applicable. Since this is a retrospective study, where only previously collected tissues were analyzed, informed consent was not required. All patients admitted to our hospital sign a consent form agreeing that their data may be used for clinical research, pending approval from the local ethics committee.
Author Contributions
Study conception and design: Ortiz-Calderon and Ruiz-Morales; data collection: Ortiz-Calderon, Arias-Ruiz, and Dorantes-Heredia; analysis and interpretation of results: Ortiz-Calderon and Ruiz-Morales; draft manuscript preparation: Ortiz-Calderon and Ruiz-Morales. All authors reviewed the results and approved the final version of the manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Niegisch G. Antibody-Drug-Conjugates (ADC): a novel treatment option in urothelial carcinoma. Methods Mol Biol. 2023;2684:293-301.
doi pubmed - Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Arch Pathol Lab Med. 2011;135(1):55-62.
doi pubmed - Meric-Bernstam F, Johnson AM, Dumbrava EEI, Raghav K, Balaji K, Bhatt M, Murthy RK, et al. Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. Clin Cancer Res. 2019;25(7):2033-2041.
doi pubmed - Bellmunt J, Werner L, Bamias A, Fay AP, Park RS, Riester M, Selvarajah S, et al. HER2 as a target in invasive urothelial carcinoma. Cancer Med. 2015;4(6):844-852.
doi pubmed - Oudard S, Culine S, Vano Y, Goldwasser F, Theodore C, Nguyen T, Voog E, et al. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur J Cancer. 2015;51(1):45-54.
doi pubmed - Powles T, Huddart RA, Elliott T, Sarker SJ, Ackerman C, Jones R, Hussain S, et al. Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2-positive metastatic bladder cancer. J Clin Oncol. 2017;35(1):48-55.
doi pubmed - Moktefi A, Pouessel D, Liu J, Sirab N, Maille P, Soyeux P, Bergman CC, et al. Reappraisal of HER2 status in the spectrum of advanced urothelial carcinoma: a need of guidelines for treatment eligibility. Mod Pathol. 2018;31(8):1270-1281.
doi pubmed - Scherrer E, Kang A, Bloudek LM, Koshkin VS. HER2 expression in urothelial carcinoma, a systematic literature review. Front Oncol. 2022;12:1011885.
doi pubmed - Wolff AC, Somerfield MR, Dowsett M, Hammond MEH, Hayes DF, McShane LM, Saphner TJ, et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-college of american pathologists guideline update. J Clin Oncol. 2023;41(22):3867-3872.
doi pubmed - Kolla SB, Seth A, Singh MK, Gupta NP, Hemal AK, Dogra PN, Kumar R. Prognostic significance of Her2/neu overexpression in patients with muscle invasive urinary bladder cancer treated with radical cystectomy. Int Urol Nephrol. 2008;40(2):321-327.
doi pubmed - Cumberbatch MGK, Jubber I, Black PC, Esperto F, Figueroa JD, Kamat AM, Kiemeney L, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74(6):784-795.
doi pubmed - Sanguedolce F, Zanelli M, Palicelli A, Bisagni A, Zizzo M, Ascani S, Pedicillo MC, et al. HER2 expression in bladder cancer: a focused view on its diagnostic, prognostic, and predictive role. Int J Mol Sci. 2023;24(4):3720.
doi pubmed - Necchi A, Giannatempo P, Paolini B, Lo Vullo S, Marongiu M, Fare E, Raggi D, et al. Immunohistochemistry to enhance prognostic allocation and guide decision-making of patients with advanced urothelial cancer receiving first-line chemotherapy. Clin Genitourin Cancer. 2015;13(2):171-177.e171.
doi pubmed - Grigg CM, Livasy C, He J, Hartman A, Clark PE, Zhu J, Raghavan D, et al. Human epidermal growth factor receptor 2 overexpression is frequently discordant between primary and metastatic urothelial carcinoma and is associated with intratumoral human epidermal growth factor receptor 2 heterogeneity. Hum Pathol. 2021;107:96-103.
doi pubmed - Li S, Wu X, Yan X, Zhou L, Xu H, Li J, et al. Prognostic value of HER2 expression levels for upper tract urothelial carcinoma. Journal of Clinical Oncology. 2022;40:557.
- Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S, Beitsch P, et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci U S A. 2004;101(25):9393-9398.
doi pubmed - Tsai YS, Tzai TS, Chow NH, Wu CL. Frequency and clinicopathologic correlates of ErbB1, ErbB2, and ErbB3 immunoreactivity in urothelial tumors of upper urinary tract. Urology. 2005;66(6):1197-1202.
doi pubmed - Indini A, Rijavec E, Grossi F. Trastuzumab Deruxtecan: changing the destiny of HER2 expressing solid tumors. Int J Mol Sci. 2021;22(9):4774.
doi pubmed - Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9-20.
doi pubmed - Venetis K, Crimini E, Sajjadi E, Corti C, Guerini-Rocco E, Viale G, Curigliano G, et al. HER2 low, ultra-low, and novel complementary biomarkers: expanding the spectrum of HER2 positivity in breast cancer. Front Mol Biosci. 2022;9:834651.
doi pubmed - Makhlin I, DeMichele A. Trastuzumab deruxtecan: an antibody-drug conjugate embracing its destiny in breast cancer. Cell Rep Med. 2022;3(6):100668.
doi pubmed - Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, Gonzalez-Martin A, Jung KH, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 Phase II trial. J Clin Oncol. 2024;42(1):47-58.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.