| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 000, Number 000, April 2025, pages 000-000
Anti-Programmed Cell Death-1 Versus Anti-Programmed Death-Ligand 1 (PD-L1) in PD-L1-Negative Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis
Laith Al-Showbakia, g, Malak Al-Kasasbehb, Karem Jbaraha, Jowan AL-Nusairc, Saif Yamind, Husam Alqaisia, Kamal Alrabie, Eitan Amirf
aDivision of Medical Oncology and Hematology, Jordan University Hospital and the School of Medicine, The University of Jordan, Amman, Jordan
bDepartment of Internal Medicine, Jordan University Hospital and the School of Medicine, The University of Jordan, Amman, Jordan
cDepartment of Internal Medicine, Joan C. Edwards School of Medicine, Marshall University, Huntington, WV, USA
dSchool of Medicine, The University of Jordan, Amman, Jordan
eDepartment of Medical Oncology, King Hussain Cancer Center, Amman, Jordan
fDivision of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University of Toronto, Ontario, Canada
gCorresponding Author: Laith Al-Showbaki, Division of Medical Oncology and Hematology, Jordan University Hospital and the School of Medicine, The University of Jordan, Amman, Jordan
Manuscript submitted January 10, 2025, accepted March 24, 2025, published online April 22, 2025
Short title: Anti-PD-1 vs. Anti-PD-L1 in PD-L1-Negative NSCLC
doi: https://doi.org/10.14740/wjon2520
| Abstract | ▴Top |
Background: Immune checkpoint inhibitors (ICIs) which target programmed cell death-1 (PD-1) receptor or its ligand (PD-L1) are used extensively in non-small cell lung cancer (NSCLC). In this article, we compared the relative efficacy of PD-1 inhibitors and PD-L1 inhibitors in PD-L1-negative advanced NSCLC.
Methods: We searched MEDLINE (host: PubMed, Scopus, and Google Scholar) for randomized trials for advanced NSCLC in which ICIs (anti-PD-1 or anti-PD-L1) were used where outcome data were reported based on PD-L1 testing, including the subset of PD-L1-negative patients. We extracted hazard ratios (HRs) and related 95% confidence intervals (CIs) and/or P values for progression-free survival (PFS) and overall survival (OS) for the PD-L1-negative subgroup of each included trial. We then pooled data using a random effects meta-analysis and compared anti-PD-1 to anti-PD-L1 inhibitors. Variations in effect size were examined using subgroup analyses.
Results: Twenty-three trials were included in the meta-analysis. PD-L1 testing was performed in all participants. A total of 4,548 PD-L1-negative patients were included in the analysis, representing 33% of all participants in the included clinical trials. Overall, the addition of anti-PD-1 was associated with better OS in PD-L1-negative advanced NSCLC patients (HR: 0.75, 95% CI: 0.67 - 0.83, P < 0.01), while the addition of anti-PD-L1 inhibitors showed no improvement in OS (HR: 0.90, 95% CI: 0.78 - 1.05, P = 0.18). Compared to anti-PD-L1 agents, anti-PD-1 resulted in better OS in PD-L1-negative patients (HR: 0.83, 95% CI: 0.67 - 0.99, P = 0.01). The differential benefit of anti-PD-1 over anti-PD-L1 was of larger magnitude when checkpoint inhibitors were used in the first-line setting (pairwise comparison HR: 0.79, 95% CI: 0.66 - 0.93, P = 0.01), while there was no difference for later lines of therapy (pairwise comparison 1.13; 95% CI: 0.82 - 1.55, P = 0.45). These differences in OS were not observed when pooling PFS data.
Conclusions: Compared to checkpoint inhibitors targeting PD-L1, those targeting PD-1 are associated with better OS in PD-L1-negative advanced NSCLC, a finding influenced by trials performed in the first-line sitting. These data should be validated using real-world studies.
Keywords: Non-small cell lung cancer; PD-L1 negative; Anti-PD-1; Anti-PD-L1; Immunotherapy
| Introduction | ▴Top |
Background
Lung cancer is a leading cause of cancer-related mortality worldwide, accounting for a significant portion of cancer deaths among both men and women [1]. Non-small cell lung cancer (NSCLC) is the most common subtype, representing approximately 85% of all lung cancer diagnoses [2]. Unfortunately, many patients are diagnosed at an advanced or unresectable stage, limiting treatment options and contributing to its poor prognosis [3]. Despite this, recent advances in immunotherapy have led to a significant improvement in mortality rates over the past 3 years [4].
A key mechanism in the treatment of NSCLC has been the understanding of how these tumors evade immune surveillance. This process is in part, driven by the interaction between the programmed death-ligand 1 (PD-L1) protein which is expressed on the surface of tumor cells and the programmed cell death-1 (PD-1) receptor found on effector T cells. This interaction effectively suppresses the cytotoxic activity of T cells, allowing tumor cells to escape immune detection and destruction. By blocking this interaction, immune checkpoint inhibitors (ICIs) disrupt the immune-suppressive environment created by the tumor during the priming phase of the cancer-immunity cycle [5]. ICIs are divided into two main subclasses: those targeting PD-1 or PD-L1 and those targeting cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) [6, 7]. PD-1 is a receptor expressed on the surface of multiple immune cell types, including T cells, B cells, and natural killer (NK) cells. One of its ligands, PD-L1, is present in different cell types including tumor cells and participates in the inhibition of previously activated T cells. CTLA-4 is present on the surface of CD4-positive and CD8-positive lymphocytes and binds to T-cell-costimulatory factors on the surface of antigen-presenting cells (APCs). CTLA-4 binding reduces interleukin-2 (IL-2) production and T-cell proliferation [6]. Checkpoint blockade using ICIs overcomes this tumor-mediated immune inhibition, leading to a proinflammatory tumor microenvironment which potentially increases anti-cancer immune effect [5, 8-10]. Recent studies focused on their role in conversion of initially unresectable advanced NSCLC into respectable disease after chemoimmunotherapy [11, 12]. Approved ICIs include anti-PD-1 antibodies (pembrolizumab, nivolumab, and cemiplimab), anti-PD-L1 (atezolizumab, avelumab, and durvalumab) and anti-CTLA-4 (ipilimumab and tremelimumab) [7].
Rationale and knowledge gap
Immunotherapy was initially approved for the subset of patients in whom the PD-L1 expression was high. Clinical evidence has shown that ICIs significantly improve outcomes for patients with advanced NSCLC when compared to traditional platinum-based chemotherapy [13]. These findings have driven the use of PD-L1 expression as a biomarker to guide treatment decisions [10, 14]. However, the clinical landscape is more complex for patients whose tumors are classified as PD-L1-negative (PD-L1 expression < 1%). Subsequent studies have shown that anti-PD-1 and anti-PD-L1 therapies can still provide benefits in PD-L1-negative NSCLC, when added to chemotherapy, in terms of both overall survival (OS) and progression-free survival (PFS) [13]. The reported prevalence of PD-L1 expression below 1% in NSCLC patients varies across clinical trials, with estimates ranging from one-third to nearly half of all cases [10]. Moreover, recent studies have highlighted the potential for combining PD-L1 inhibitors with other treatments, such as bevacizumab and chemotherapy, to enhance efficacy without significantly increasing adverse events [8].
Despite these promising findings, questions remain regarding the differential efficacy of anti-PD-1 and anti-PD-L1 agents in PD-L1-negative NSCLC patients. While both classes of ICIs target the same immune pathway, they have distinct mechanisms of action and pharmacological properties [14].
Objective
It is still unclear whether one class of drugs offers superior outcomes in PD-L1-negative patients. Furthermore, while no significant differences in side effects have been observed between these therapies in small cell lung cancer [15], the impact of these therapies in PD-L1-negative NSCLC patients remains underexplored.
This meta-analysis aimed to study the relative efficacy of PD-L1 inhibitors and PD-1 inhibitors in PD-L1-negative advanced NSCLC patients, which could help inform treatment decisions and guide future research efforts in developing more effective, personalized treatment strategies for patients with advanced NSCLC.
The systematic review and meta-analysis study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2020 guidelines [16].
| Materials and Methods | ▴Top |
Literature review and study identification
A systematic search of published randomized controlled trials (RCTs) was conducted in July 2024 using MEDLINE (host: PubMed, Scopus, and Google Scholar). Inclusion criteria comprised RCTs of any phase involving patients with advanced NSCLC in which participants received either anti-PD-1 (pembrolizumab, nivolumab, toripalimab, cemiplimab, tislelizumab, and camrelizumab) or anti-PD-L1 (atezolizumab, duravlumab, and avelumab) either alone or in combination with other systemic therapy against a respective standard of care therapy (SOC), availability of outcome data; namely progression-free survival (PFS) and/or overall survival (OS) reported based on PD-L1 expression status; specifically for the subset of patients with negative PD-L1 expression. Ongoing trials, trials including only patients with positive PD-L1 expressions, and trials which did not include the prespecified reported outcomes were excluded from this study. When several versions of the same RCTs were identified, we included the most recent version that included the predefined outcome data. Each study was screened separately by two independent investigators. Discrepancy in date was resolved by a third investigator. Institutional Review Board approval is not applicable for systematic review and meta-analysis.
Data collection
After RCTs were identified, data extraction was performed by two independent investigators. Extracted data comprised the name and the date of publication of each included trial, the histological subtype (squamous vs. non-squamous), treatment line (first line vs. later line), drug subclass (anti-PD-1 vs. anti-PD-L1), experimental arm and control arm of each included trial, the sample size of the PD-L1-negative cohort, and the total sample size of each included trial. For outcome measures, we extracted the hazard ratio (HR) and related 95% confidence intervals (CIs) for PFS and/or OS for the subset of patients who had negative PD-L1 expression as defined by each individual trial.
Data synthesis and statistical analysis
The HRs for PFS and OS for the PD-L1-negative cohort of patients in each included study were pooled using the generic inverse variance. Random effect modeling was utilized due to both clinical and statistical heterogeneity amongst included trials. Comparisons between PD-1 and PD-L1 inhibitors were performed using subgroup analysis as described by Deeks [17]. Analyses were performed using Review Manager V.5.4.1 (The Cochrane Collaboration, Copenhagen, Denmark). Variation in effect size was examined using subgroup analyses; specifically, we assessed the association of effect size with treatment line (first line vs. later line), histological subtype (squamous vs. non-squamous), and according to the used anti-PD-1/PD-L1 agent (pembrolizumab, nivolumab, atezolizumab, and durvalumab). Pairwise comparisons of pooled data were then performed using WINBUGS within Microsoft Excel (Microsoft Corp., Redmond, WA, USA). Statistical significance was defined as P < 0.05. We did not apply corrections for multiple significance testing.
| Results | ▴Top |
The study selection scheme is shown in Figure 1. Twenty-three trials were included in the meta-analysis. PD-L1 testing was performed in all participants, where a total of 4,548 PD-L1-negative patients were identified in this study, representing 33% of all participants in included clinical trials. The platforms used for PD-L1 testing and the expression used to describe the population of patients who showed no PD-L1 expression varied amongst the trials involving different agents; however, PD-L1-negative tumors were defined in all included trials as the tumors that do not explicit any inducible expression of PD-L1 by tumor or immune infiltrating cell.
 Click for large image | Figure 1. Study selection schema. |
OS data were available from 21 out of the 23 included trials, while PFS data were available from 20 trials. Amongst the included trials, 18 trials were undertaken in the first-line sitting, of which 11 trials included anti-PD-1. Twelve trials included patients with non-discriminatory NSCLC histological subtypes. Out of the remaining 11 trials, seven were devoted to the non-squamous subtype. Characteristics of included studies are reported in Table 1 [18-40].
 Click to view | Table 1. Characteristics of Included Clinical Trials |
Funnel plot to assess publication bias is demonstrated in Figure 2a, b. Heterogeneity of the samples of the 14 studies that included anti-PD-1 was tested using Chi-square (Q test = 9.21, df = 11, P = 0.60), which means no significant heterogeneity as demonstrated in Figure 2c. On the other hand, heterogeneity of the nine trials studying PD-L1 testing (Chi-square (Q test) = 15.56, df = 8, P = 0.05) was significant as demonstrated in Figure 2d.
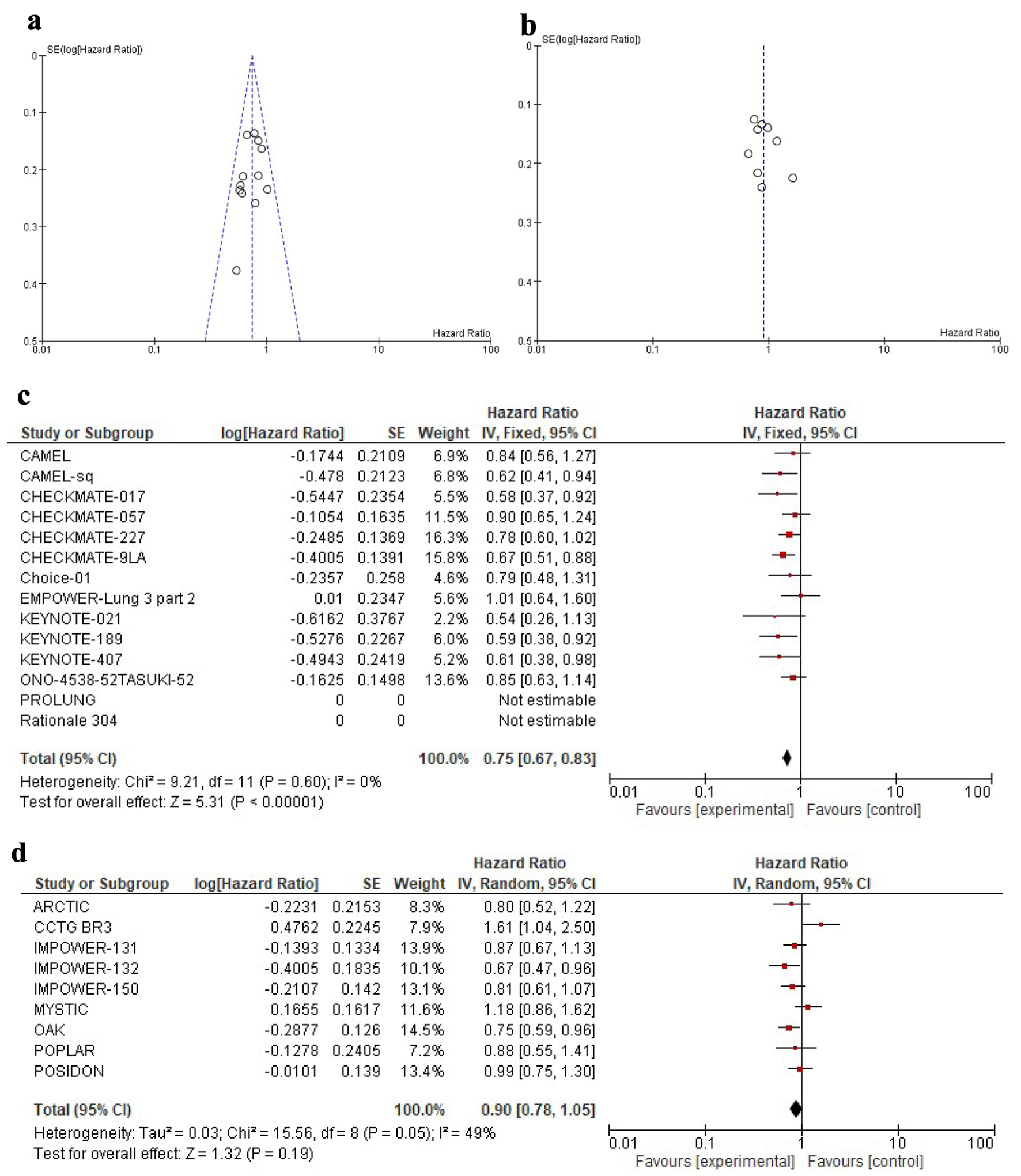 Click for large image | Figure 2. Funnel plot comparing the precision of individual studies in (a) anti-PD-1 and (b) anti-PD-L1. The y-axis represents the standard error. The x-axis displays the study estimated effect size (hazard ratio). Forrest plot comparing the heterogeneity of the included trials for the two subclasses (c) anti-PD-1 and (d) anti-PD-L1.PD-1: programmed cell death-1; PD-L1: programmed death-ligand 1. |
A summary of the main and subgroup data results is shown in Tables 2 and 3.
 Click to view | Table 2. Results of the Differential Efficacy Between Anti-PD-1 Over Anti-PD-L1 in PD-L1-Negative Advanced NSCLC Patients |
 Click to view | Table 3. Association Between Histological Subtype and Outcomes |
Main analysis (comparison of PD-1 to PD-L1 inhibitors)
Overall, the addition of anti-PD-1 was associated with better OS in PD-L1-negative advanced NSCLC patients (HR: 0.75, 95% CI: 0.67 - 0.83, P < 0.01) as shown in Figure 2c, while the addition of anti-PD-L1 inhibitors showed neither a significant nor meaningful improvement in OS (HR: 0.90, 95% CI: 0.78 - 1.05, P = 0.18) as shown in Figure 2d. Compared to anti-PD-L1 agents, anti-PD-1 inhibitors showed better OS in PD-L1-negative patients (HR: 0.83, 95% CI: 0.67 - 0.99, P = 0.01).
For PFS, pooling of individual trials showed a higher magnitude of effect for PD-1 compared to PD-L1 inhibitors (HR: 0.69, 95% CI: 0.63 - 0.76, P < 0.01 versus HR: 0.83, 95% CI: 0.74 - 0.94, P < 0.01). Again, these differences were statistically significant in pairwise comparisons (HR: 0.83, 95% CI: 0.71 - 0.97, P = 0.02).
Subgroup analyses
Association between the line of therapy and OS
The differential benefit of anti-PD-1 over anti-PD-L1 inhibitors was found to be more pronounced if the agents were used in the first-line setting (pairwise comparison HR: 0.79, 95% CI: 0.66 - 0.93, P = 0.01). In contrast, there was no statistically significant difference in OS between the two subclasses if used during the later lines of therapy (pairwise comparison 1.13; 95% CI: 0.82 - 1.55, P = 0.45) as shown in Table 2.
Association between the line of therapy and PFS
Similar trends albeit with lower magnitude were observed for the PFS endpoint. There was a non-significant association with greater improvement in PFS with PD-1 inhibitors compared with PD-L1 inhibitors when used in the first line (pairwise comparison HR: 0.84, 95% CI: 0.68 - 1.03, P = 0.09). No effect was seen for later lines (pairwise HR: 1.06, 95% CI: 0.78 - 1.43, P = 0.70) as shown in Table 2.
Association between histological subtype and outcome
Among trials that explored specific histological subtypes of NSCLC, the addition of anti-PD-1 agents was associated with a significant improvement in OS among the two histological subtypes, squamous (HR: 0.60, 95% CI: 0.47 - 0.78, P < 0.01) and non-squamous (HR: 0.79, 95% CI: 0.65 - 0.95, P = 0.01). Conversely, the utilization of anti-PD-L1 agents showed non-significant effects in the two histological subtypes (HR: 0.87, 95% CI: 0.67 - 1.13 in squamous and HR: 0.81, 95% CI: 0.61 - 1.07 in non-squamous) as shown in Table 3. Due to the small number of studies available for review, pairwise comparisons were not performed.
| Discussion | ▴Top |
Based on the results of multiple landmark trials, ICIs were approved as monotherapy, in PD-L1 high NSCLC patients, or combined with chemotherapy for the remainder of patients [18]. Several trials were conducted to examine the benefit of adding anti-PD-1 or anti-PD-L1 into the treatment paradigm for PD-L1-negative NSCLC [13], a group which represents a considerable proportion of the whole patients’ spectrum. Both anti-PD-1 and anti-PD-L1 have different specific properties in terms of binding to different targets, where efficacy may vary based on the site of inhibition [7]. However, little is known about the differential efficacy of one class over the other.
Key findings
In our meta-analysis comparing the efficacy of anti-PD-1 agents with that of anti-PD-L1 agents, we observed a significantly greater magnitude of improved OS with anti-PD-1 compared with anti-PD-L1. This effect appeared to apply only to treatment delivered in the first-line sitting. We also extended the comparison to cover the different histological subtypes (when reported), and this analysis proved a consistent differential benefit of anti-PD-1 over anti-PD-L1 independent of the histological subtype. Of note, while effect sizes were in a similar direction for PFS, differences were not statistically significant.
Strengths and limitations
This study provides clinicians with valuable insights into which ICI class is more effective in first-line settings in PD-L1-negative NSCLC by observing a statistically significant OS benefit with anti-PD-1 therapies, which is an impactful endpoint even if PFS differences were not significant. Moreover, by examining the impact on various histological subtypes, generalizability of the findings is further enhanced.
This study has limitations. First, the relatively small number of included trials may have an impact on the accuracy of our analysis and will have reduced power to observe statistically significant results especially for some subgroup analyses. In addition, in all allocated trials, anti-PD-1/ PD-L1 therapies were used either alone or in combination with SOC systemic therapies comprising different forms of chemotherapeutic agents. Thus, a significant degree of heterogeneity between included interventions was noted. We did utilize methods to address this heterogeneity (e.g., random effects models), but residual uncertainty will likely remain. Third, a degree of selection bias is expected as we could not include all approved anti-PD-1/PD-L1 in this analysis. For example, the efficacy of avelumab (anti-PD-L1) was only studied in the PD-L1-positive patients and therefore, there were no available outcome data for the PD-L1-negative advanced NSCLC patients. Moreover, recent studies have increasingly emphasized the use of anti-PD-1 therapies rather than anti-PD-L1, which could potentially influence the outcomes of this metanalysis. However, despite these limitations, this study may identify future research in the same field studying the differences between anti-PD-1 and anti-PD-L1, not just in NSCLC, but in other disease sites where both PD-1 and PD-L1 inhibitors are in use.
Comparison with similar research
The efficacy of both ICI subclasses targeting PDL pathway was previously studied regardless of PD-L1 expression in advanced NSCLC, particularly in squamous and low PD-L1 expression. Possible interclass differences favoring anti-PD-1 was highlighted [41]. Also, differences between the two subclasses were studied in the setting of head and neck squamous cell carcinoma (SCC) without the clarification of PDL receptor status and there was a significant difference between the two favoring anti-PD-1 in metastatic disease in which anti-PD-1 lowered mortality [42]. However, up until this meta-analysis, little was known about their relative effect in PD-L1-negative advanced NSCLC patients and their different subclasses.
Explanations of findings
The discordance between PFS and OS is observed occasionally with ICIs and other treatments that may affect tumor growth kinetics instead of imposing a direct cytotoxic effect on cancer cells, which may hinder the observed benefits in PFS while the differential benefit in OS is still significant [43]. This includes the potential observation of pseudo-progression which may impact on PFS data. The US Food and Drug Administration (FDA) has examined several trials in which an OS benefit was demonstrated without significant improvements in intermediate endpoints (PFS or response rate) after which several ICIs were granted approvals for NSCLC, head and neck SCC, and melanoma [43-47]. It is noteworthy that PD-1 receptor expressed on immune cells has two ligands (PD-L1/PD-L2). The precise role of PD-L2 in cancer immunity remains incompletely understood. However, unlike anti-PD-L1, anti-PD-1 neutralizes both PD-L1 and PD-L2 [19-21], and this might explain the improved differential efficacy in anti-PD-1 over anti-PD-L1. Among the 23 trials analyzed, the POPLAR trial was the only study to assess PD-L2 status and atezolizumab (an anti-PD-L1) was still effective in tumors with high expressions of PD-L2 [22].
Implications and actions needed
Further studies with larger sample size might add a huge value to the generalizability of the conducted results, especially those studying the effect of ICIs in PD-L1-negative advanced NSCLC in the first line without chemoradiation that might provide some answers to the real effect without any confounders either by seroconversion to PD-L1-positive after chemoradiation [48], or the lack of difference in PFS which was partially justified by pseudoefficacy. Also, inclusion of emerging studies that highlights the previously non-studied ICIs of PD-L1-negative NSCLC will add further external validity to the results.
Finally, studying PD-L2 status and investigating other potential biomarkers that contribute to tumor microenvironment and immune escape, including tumor mutational burden (TMB) which correlates with the response to anti-PD-1/anti-PD-L1 [49, 50], interferon (IFN)-γ-related gene signatures in non-resectable melanoma although studies failed to correlate the level in NSCLC [51], and STK11/KEAP1 mutations which correlate with poor response to anti-PD-1 and anti-PD-L1 [52]. Decline in the level of circulating tumor DNA (ctDNA) in advanced solid tumors is associated with substantial improvement in outcome and response and might guide treatment plans [53], and finally, the concept of heterogeneity immune checkpoint signature (HIS) which might explain the difference in response to ICIs according to the size of the solid tumor [54].
Conclusions
In summary, among patients with PD-L1-negative advanced NSCLC, it appears that anti-PD-1 inhibitors are associated with greater magnitude improvement in OS compared to anti-PD-L1 inhibitors, especially if used as first-line treatment. There was no apparent influence of histological subtype. The lack of confirmatory effect on PFS means that these data need to be validated, preferably in large real-world studies.
Learning points
Anti-PD-1 therapies are associated with better OS than anti-PD-L1 in PD-L1-negative advanced NSCLC, a finding influenced by the included trials which were performed in the first-line sitting.
This analysis proved a consistent differential benefit of anti-PD-1 over anti-PD-L1 independent of the histological subtype in advanced patients with PD-L1-negative NSCLC. Of note, while effect sizes were in a similar direction for PFS, differences were not statistically significant.
These results could help inform treatment decisions and guide future research efforts in developing more effective, personalized treatment strategies for patients with advanced NSCLC.
Acknowledgments
We would like to thank the reviewers for all the valuable comments and suggestions which helped us to improve the quality of the manuscript.
Financial Disclosure
None to declare.
Conflict of Interest
Laith Al-Showbaki: consulting from Novartis, AstraZeneca, and Janssen. Eitan Amir: consulting from Gilead and Novartis.
Informed Consent
Not applicable.
Author Contributions
Conceptualization: Laith Al-Showbaki. Data curation: Malak Al-Kasasbeh, Karem Jbarah, and Saif Yamin. Formal analysis: Laith Al-Showbaki. Methodology: Laith Al-Showbaki. Writing - original draft: all authors. Writing - review and editing: all authors.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. Epidemiology of lung cancer. Contemp Oncol (Pozn). 2021;25(1):45-52.
doi pubmed - Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288-300.
doi pubmed - Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, Petrella F, et al. Non-small-cell lung cancer. Nat Rev Dis Primers. 2015;1:15009.
doi pubmed - Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, Mariotto AB, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640-649.
doi pubmed - Onoi K, Chihara Y, Uchino J, Shimamoto T, Morimoto Y, Iwasaku M, Kaneko Y, et al. Immune checkpoint inhibitors for lung cancer treatment: a review. J Clin Med. 2020;9(5):1362.
doi pubmed - Mamdani H, Matosevic S, Khalid AB, Durm G, Jalal SI. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front Immunol. 2022;13:823618.
doi pubmed - Olivares-Hernandez A, Gonzalez Del Portillo E, Tamayo-Velasco A, Figuero-Perez L, Zhilina-Zhilina S, Fonseca-Sanchez E, Miramontes-Gonzalez JP. Immune checkpoint inhibitors in non-small cell lung cancer: from current perspectives to future treatments-a systematic review. Ann Transl Med. 2023;11(10):354.
doi pubmed - Ge Y, Zhan Y, He J, Li J, Wang J, Wei X, Wang C, et al. PD-(L)1 inhibitors plus bevacizumab and chemotherapy as first-line therapy in PD-L1-negative metastatic lung adenocarcinoma: a real-world data. J Cancer Res Clin Oncol. 2024;150(3):135.
doi pubmed - Ferrara R, Imbimbo M, Malouf R, Paget-Bailly S, Calais F, Marchal C, Westeel V. Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2020;12(12):CD013257.
doi pubmed - Dietel M, Savelov N, Salanova R, Micke P, Bigras G, Hida T, Antunez J, et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: The global, multicenter EXPRESS study. Lung Cancer. 2019;134:174-179.
doi pubmed - Yi C, Bian D, Wang J, Hu S, Sun L, Yan Y, Wang S, et al. Anti-PD1 based precision induction therapy in unresectable stage III non-small cell lung cancer: a phase II umbrella clinical trial. Nat Commun. 2025;16(1):1932.
doi pubmed - Zhu X, Sun L, Song N, He W, Xie B, Hu J, Zhang J, et al. Safety and effectiveness of neoadjuvant PD-1 inhibitor (toripalimab) plus chemotherapy in stage II-III NSCLC (LungMate 002): an open-label, single-arm, phase 2 trial. BMC Med. 2022;20(1):493.
doi pubmed - Tostes K, Siqueira AP, Reis RM, Leal LF, Arantes L. Biomarkers for immune checkpoint inhibitor response in NSCLC: current developments and applicability. Int J Mol Sci. 2023;24(15):11887.
doi pubmed - Surmiak E, Magiera-Mularz K, Musielak B, Muszak D, Kocik-Krol J, Kitel R, Plewka J, et al. PD-L1 inhibitors: different classes, activities, and mechanisms of action. Int J Mol Sci. 2021;22(21):4925-4937.
doi pubmed - Yang G, Sun H, Sun N, Huang W, Wang Z, Zhang H, Liu C. Efficacy and safety comparison of PD-1 inhibitors vs. PD-L1 inhibitors in extensive-stage small-cell lung cancer: a retrospective comparative cohort study. J Thorac Dis. 2022;14(12):4925-4937.
doi pubmed - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed - Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323(7305):157-162.
doi pubmed - Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031.
doi pubmed - Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135.
doi pubmed - Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497-1508.
doi pubmed - Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, Lee KH, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2021;32(9):1137-1147.
doi pubmed - Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846.
doi pubmed - Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092.
doi pubmed - Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Hermes B, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040-2051.
doi pubmed - Planchard D, Reinmuth N, Orlov S, Fischer JR, Sugawara S, Mandziuk S, Marquez-Medina D, et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol. 2020;31(5):609-618.
doi pubmed - Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, van den Heuvel MM, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020;6(5):661-674.
doi pubmed - Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, Kim SW, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol. 2023;41(6):1213-1227.
doi pubmed - Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639.
doi pubmed - Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, Soo R, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15(8):1351-1360.
doi pubmed - Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301.
doi pubmed - Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265.
doi pubmed - Arrieta O, Barron F, Ramirez-Tirado LA, Zatarain-Barron ZL, Cardona AF, Diaz-Garcia D, Yamamoto Ramos M, et al. Efficacy and safety of pembrolizumab plus docetaxel vs docetaxel alone in patients with previously treated advanced non-small cell lung cancer: the PROLUNG phase 2 randomized clinical trial. JAMA Oncol. 2020;6(6):856-864.
doi pubmed - Leighl NB, Laurie SA, Goss GD, Hughes BGM, Stockler M, Tsao MS, Hwang DM, et al. CCTG BR34: a randomized phase 2 trial of durvalumab and tremelimumab with or without platinum-based chemotherapy in patients with metastatic NSCLC. J Thorac Oncol. 2022;17(3):434-445.
doi pubmed - Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198-211.
doi pubmed - Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, Longeras PD, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16(4):653-664.
doi pubmed - Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, Han L, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non-small-cell lung cancer: a multicenter randomized phase III trial (CHOICE-01). J Clin Oncol. 2023;41(3):651-663.
doi pubmed - Makharadze T, Gogishvili M, Melkadze T, Baramidze A, Giorgadze D, Penkov K, Laktionov K, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in advanced NSCLC: 2-year follow-up from the phase 3 EMPOWER-Lung 3 part 2 trial. J Thorac Oncol. 2023;18(6):755-768.
doi pubmed - Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, Ma Z, et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): a randomized phase 3 trial. J Thorac Oncol. 2021;16(9):1512-1522.
doi pubmed - Xie Q, Zheng H, Su N, Li Q. Camrelizumab in patients with advanced non-squamous non-small cell lung cancer: a cost-effective analysis in China. BMJ Open. 2022;12(8):e061592.
doi pubmed - Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, Pan Y, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): a phase 3 trial. J Thorac Oncol. 2022;17(4):544-557.
doi pubmed - Banna GL, Cantale O, Bersanelli M, Del Re M, Friedlaender A, Cortellini A, Addeo A. Are anti-PD1 and anti-PD-L1 alike? The non-small-cell lung cancer paradigm. Oncol Rev. 2020;14(2):490.
doi pubmed - Botticelli A, Cirillo A, Strigari L, Valentini F, Cerbelli B, Scagnoli S, Cerbelli E, et al. Anti-PD-1 and Anti-PD-L1 in Head and Neck Cancer: A Network Meta-Analysis. Front Immunol. 2021;12:705096.
doi pubmed - Merino M, Kasamon Y, Theoret M, Pazdur R, Kluetz P, Gormley N. Irreconcilable differences: the divorce between response rates, progression-free survival, and overall survival. J Clin Oncol. 2023;41(15):2706-2712.
doi pubmed - Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro Carpeno J, Pluzanski A, et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39(7):723-733.
doi pubmed - Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr., Psyrri A, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928.
doi pubmed - Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
doi pubmed - Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Jr., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830.
doi pubmed - Wang Y, Kim TH, Fouladdel S, Zhang Z, Soni P, Qin A, Zhao L, et al. PD-L1 expression in circulating tumor cells increases during radio(chemo)therapy and indicates poor prognosis in non-small cell lung cancer. Sci Rep. 2019;9(1):566.
doi pubmed - Andrews MC, Li G, Graf RP, Fisher VA, Mitchell J, Aboosaiedi A, O'Rourke H, et al. Predictive impact of tumor mutational burden on real-world outcomes of first-line immune checkpoint inhibition in metastatic melanoma. JCO Precis Oncol. 2024;8:e2300640.
doi pubmed - Vryza P, Fischer T, Mistakidi E, Zaravinos A. Tumor mutation burden in the prognosis and response of lung cancer patients to immune-checkpoint inhibition therapies. Transl Oncol. 2023;38:101788.
doi pubmed - Dizier B, Callegaro A, Debois M, Dreno B, Hersey P, Gogas HJ, Kirkwood JM, et al. A Th1/IFNgamma gene signature is prognostic in the adjuvant setting of resectable high-risk melanoma but not in non-small cell lung cancer. Clin Cancer Res. 2020;26(7):1725-1735.
doi pubmed - Papillon-Cavanagh S, Doshi P, Dobrin R, Szustakowski J, Walsh AM. STK11 and KEAP1 mutations as prognostic biomarkers in an observational real-world lung adenocarcinoma cohort. ESMO Open. 2020;5(2):e000706.
doi pubmed - Al-Showbaki L, Wilson B, Tamimi F, Molto C, Mittal A, Cescon DW, Amir E. Changes in circulating tumor DNA and outcomes in solid tumors treated with immune checkpoint inhibitors: a systematic review. J Immunother Cancer. 2023;11(2):e005854.
doi pubmed - Fan P, Qi Z, Liu Z, Wang S, Wang Y, Kuai J, Zhang N, et al. High baseline levels of PD-L1 reduce the heterogeneity of immune checkpoint signature and sensitize anti-PD1 therapy in lung and colorectal cancers. Cell Death Dis. 2025;16(1):152.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.