| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 6, December 2024, pages 922-928
Causal Relationships Between Gut Microbiota, Immune Cell and Pancreatic Cancer: A Two-Step, Two-Sample Mendelian Randomization Study
Si Ming Wanga, e, Ming Feng Zhangb, e, Qian Hui Panc, e, Ting Feng Yua, c, Rui Lin Leia, d, f, Qing Jian Lia, b, f
aGuangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China
bDepartment of Oncology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China
cDepartment of Gastroenterology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China
dDepartment of Gynecological Oncology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China
eThese authors contributed equally to this article.
fCorresponding Author: Qing Jian Li, Department of Oncology and Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China; Rui Lin Lei, Department of Gynecological Oncology and Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China
Manuscript submitted August 25, 2024, accepted October 17, 2024, published online October 30, 2024
Short title: Causal Relation Between GM, Immune Cell and PC
doi: https://doi.org/10.14740/wjon1960
| Abstract | ▴Top |
Background: Gut microbiota (GM) is associated with both the occurrence and development of pancreatic cancer (PC), and immune cells potentially play a role in this process. This study sought to evaluate the causative effect of GM on PC and to ascertain possible immune cell mediators.
Methods: The study primarily employed a two-step, two-sample Mendelian randomization (MR) analysis to explore the causal relationship between GM and PC within the European population, placing particular emphasis on the application of the inverse variance weighted (IVW) approach. Additionally, mediation analysis was conducted to explore the potential influence of immune cells as mediators.
Results: The MR analysis revealed a significant association between Geminocystis and the risk of PC. Increased abundance of Geminocystis was positively associated with the risk of PC (odds ratio (OR): 2.580, 95% confidence interval (CI): 1.050 - 6.342). The validity of the outcomes was also verified by the sensitivity analysis. The mediation MR analysis showed that the B-cell absolute count served as a partial intermediary in the causal link between Geminocystis and the risk of PC, contributing to 15.321% of the mediating impact.
Conclusion: This MR study demonstrated that Geminocystis has a causal relationship with PC and potentially mediates B-cell absolute count in the TBNK panel.
Keywords: Gut microbiota; Immune cell; Pancreatic cancer; Mendelian randomization
| Introduction | ▴Top |
Pancreatic cancer (PC) is frequently identified as a common malignant neoplasm in the digestive system across numerous countries, and it is characterized as a lethal form of cancer [1]. Data have shown that PC ranks as the third highest cause of cancer deaths in the United States, and its occurrence continues to increase annually. Despite advancements in PC treatment strategies such as targeted therapy and immunotherapy, the overall prognosis of PC remains unfavorable, with a 5-year survival rate of approximately 7.2% [2]. Additionally, the efficacy of various immunotherapies for PC treatment has been less than optimal [3]. Most PC patients do not respond to single immune checkpoint blockade therapy, and the targeted mesothelin-directed chimeric antigen receptor (CAR) T-cell therapy shows limited effectiveness in PC, failing to achieve the expected results [4, 5]. PC has become a major public health issue threatening public health. Accordingly, it is imperative to delve into the processes that drive the onset and escalation of PC and to promote updates in prevention, diagnosis, and treatment methods for this disease.
The gut microbiota (GM) forms a substantial and intricate microbial community that is intimately linked to the well-being of the host. The GM generates metabolites such as short-chain fatty acids (SCFAs), tryptophan, and bile acid derivatives, which influence not just genetic and epigenetic controls but also the metabolic activities of immune cells, including those with immunosuppressive and inflammatory functions [6]. Enhancing evidence suggests that the composition and distribution of GM vary across different cancers, and an altered GM profile can potentially result in the occurrence of various diseases, including PC, colorectal tumors, and lung cancer. Research indicates that butyrate, a metabolic product of the GM, can delay the progression of PC by neutralizing the immunosuppressive effects of CD11b cells and enhancing the immunological responses of CD8+ lymphocytes [6, 7]. Thus, the GM not only affects nutritional absorption and metabolism but also co-evolves with the host’s immune system to form a complex interplay.
Mendelian randomization (MR) serves as an analytical technique used to explore causal relationships and ascertain the linkage between an exposure and its subsequent outcome [8]. Previous evidence has extensively confirmed the role of GM in PC and its impact on people’s immunological system [9]. Yet, whether GM affects the progression of PC through immune cell regulation remains unclear. This research is designed to probe the causal relationships between GM and PC, as well as to determine whether immune cells may mediate this process. The MR method employs genetic variation as an instrumental variable (IV) to infer causal impacts, which, in theory, bypasses the effects of confounding elements or the issue of reverse causality. Two-sample MR analysis is a typical MR method and can be used to access causal connections from two independent samples. In this study, we investigated the influence of GM on immune cell properties and computed the percentage of the impact of GM on PC that is mediated through immune cell features, in order to determine whether GM can affect PC by regulating immunological system.
| Materials and Methods | ▴Top |
Study design
This study aimed to evaluate the causal relationship from GM to PC through immune cells using genetic instruments. The detailed layout and procedural sequence of this MR study consisted of two principal elements: 1) the examination of the causal impacts of 473 GM on PC, as well as the assessment of the causal influences exerted by 731 immune-related genomic characteristics on PC; 2) and mediation analysis of 731 immune-related whole-genome features in the pathway from GM to PC. The overview of research design is illustrated in Figure 1.
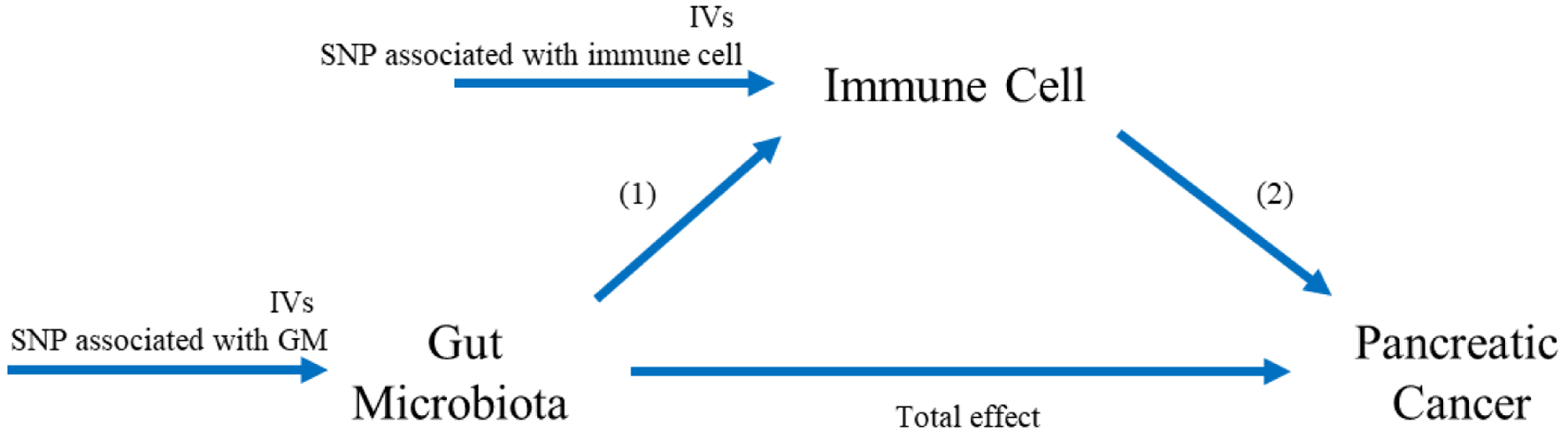 Click for large image | Figure 1. The study design. A two-step Mendelian randomization study of GM on PC mediated by immune cell. IVs: instrumental variables; SNP: single nucleotide polymorphism. |
In the MR analysis, three key assumptions must be satisfied to yield valid results: 1) The IVs must exhibit a robust and reliable correlation with the exposure under investigation. 2) The IVs should be unrelated to any confounders that are associated with both genetic variants and the outcome being studied. 3) The IVs must affect the outcome solely via their association with the exposure, excluding any additional intermediary processes [10]. The STROBE-MR checklist of the study is shown in Supplementary Material 1 (wjon.elmerpub.com).
Data sources
The datasets utilized in this research were accessible to the general public, and the individuals involved in the database were of European descent. The genetic associations of GM were derived from the NHGRI-EBI GWAS Catalog from accession GCST90032172 to GCST90032644 [11].
Access to the immune cells data is unrestricted through the GWAS Catalog, with a range of accession numbers from GCST0001391 to GCST0002121 [12]. This dataset consists of 731 immunophenotypic measurements including six panels: TBNK (B cells, natural killer (NK) cells, T cells), T regulatory cells, myeloid cells, maturation stages of T cells, B cells and conventional dendritic cells (CDCs).
For the outcome dataset, GWAS summary statistics for PC were sourced from the FinnGen Consortium R10 release. This study used the “adenocarcinoma and ductal carcinoma of pancreas” phenotype and included 314,924 participants (731 PC cases and 314,193 controls).
Statistical analysis
Significant single nucleotide polymorphisms (SNPs) at the threshold P < 1 × 10-5 were recognized as potential IVs [13]. To mitigate bias arising from substantial linkage disequilibrium (LD), we removed SNPs with an r2 < 0.001 from a 10,000 kb window. To avoid the bias of weak IVs, we calculated R2 and F statistics of the selected SNPs. The methods of calculation for R2 and F statistics were used as previously reported [14, 15]. The equation for R2 was: (2 × Effect2 × eaf × (1 - eaf))/(2 × Effect2 × eaf × (1 - eaf) + se2 × 2 × N × eaf × (1 - eaf)). The F statistic was calculated as: (R2 × (N - 2)/(1 - R2)). R2 represented the proportion of GM variance accounted for by genetic instruments, and we excluded SNPs with F statistics below 10. The minimal allele frequency (MAF) was set to 0.01 and we decided not to employ the SNP proxy [16]. After integrating the effect alleles from the GWAS studies of exposure and outcome, the causality between GM and PC was estimated with several MR approaches, including the IVW, weighted median, MR-Egger and weighted mode. Among them, the IVW method was considered the main outcome, which can provide the most accurate assessments [17]. Sensitivity analysis has played a crucial role in MR analysis to evaluate potential pleiotropy, and the heterogeneity for MR estimations can be seriously violated. The heterogeneity of the study was quantified by Cochran’s Q test. We applied the MR-Egger intercept and MR-PRESSO to determine whether pleiotropy existed [18]. Leave-one-out analysis was applied to examine whether the MR results were affected by singular SNP. We conducted MR analysis using R software (version 4.4.0) [19] along with the “Two-Sample MR” package (version 0.6.3). P < 0.05 was considered to denote statistical significance.
This study is a secondary analysis conducted through existing GWAS data. No ethics approval was needed due to the re-analysis of published data.
| Results | ▴Top |
Association of GM with PC
The IVW, weighted median, MR-Egger and weighted mode were used to estimate the causal relationship between genetically predicted GM and PC. Detailed information on SNPs included for analyses is presented in Supplementary Material 2 (wjon.elmerpub.com). The two-sample MR analysis demonstrated the causality of Geminocystis on PC. While alternative methods did not indicate statistically significant results, the IVW strategy disclosed that elevated levels of Geminocystis corresponded with an escalated probability of developing PC (odds ratio (OR): 2.580, 95% confidence interval (CI): 1.050 - 6.342, P = 0.039) (Fig. 2). The statistical methods of Cochran’s Q, the MR-Egger intercept test, and MR-PRESSO indicated no presence of heterogeneity and horizontal pleiotropy within the context of the MR study (Supplementary Material 3, wjon.elmerpub.com). Furthermore, during a leave-one-out sensitivity analysis, no SNP significantly altered the consistent relationship between GM and the incidence of PC (Fig. 3a). Similarly, the reverse MR analysis failed to detect any reverse causation (Supplementary Material 4, wjon.elmerpub.com).
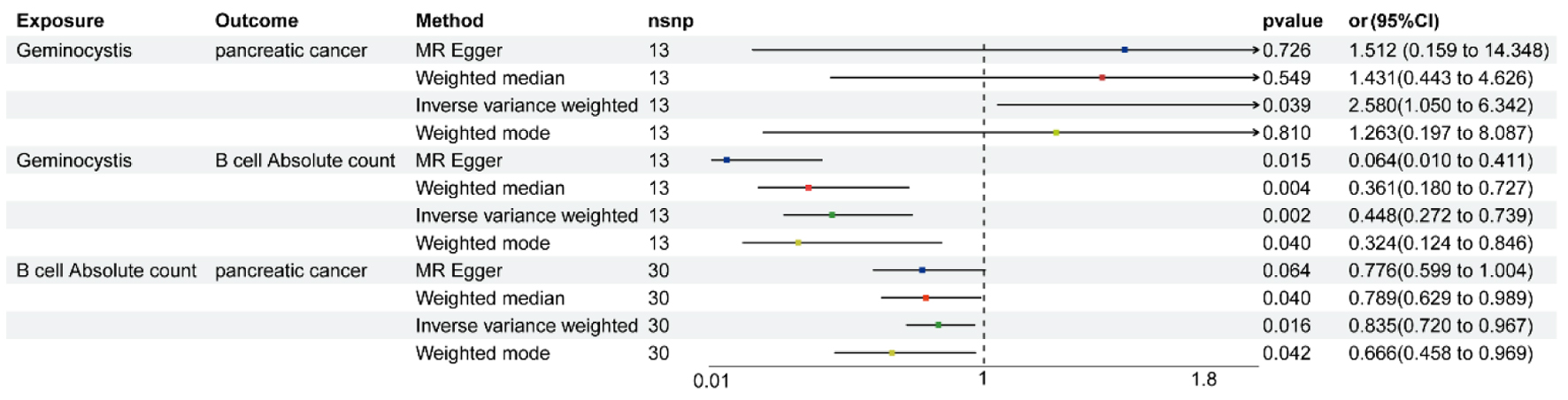 Click for large image | Figure 2. MR analysis showing the causality of Geminocystis on pancreatic cancer is significant. MR: Mendelian randomization; CI: confidence interval; OR: odds ratio; SNP: single nucleotide polymorphism. |
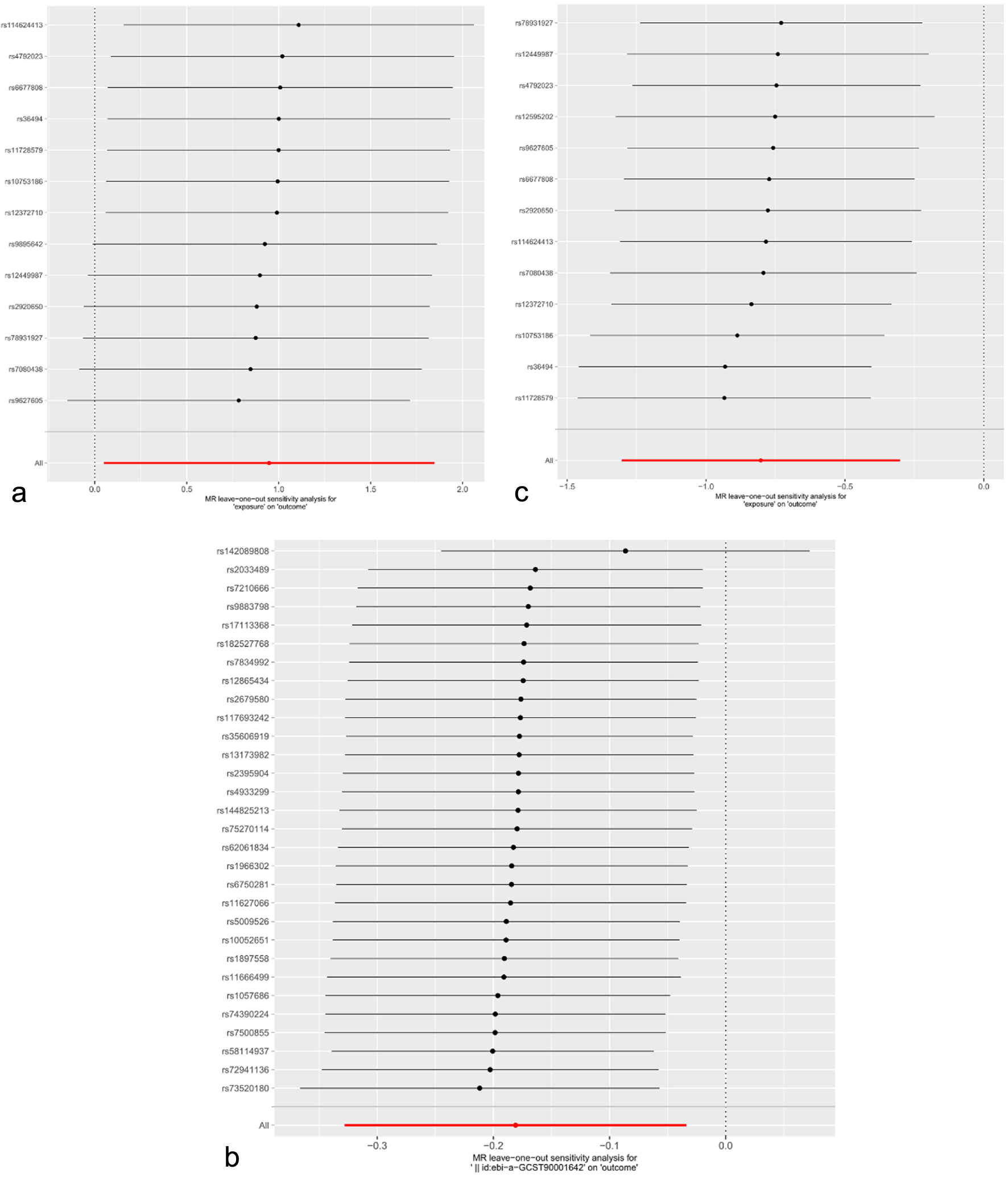 Click for large image | Figure 3. Leave-one-out plots for MR analyses. (a) The causal effect of GM on PC. (b) The causal effect of immune cell on PC. (c) The causal effect of GM on immune cell. SNPs: single nucleotide polymorphisms; MR: Mendelian randomization; GM: gut microbiota; PC: pancreatic cancer. |
Association of immune cell with PC
Protective effect of B-cell absolute count (AC) traits on PC was identified with the IVW, weighted median, MR-Egger and weighted mode (Fig. 1, Supplementary Material 5, wjon.elmerpub.com). No apparent heterogeneity and horizontal pleiotropy were found in the current study (Supplementary Material 3, wjon.elmerpub.com). The leave-one-out sensitivity analysis substantiated the stability of the findings, demonstrating that the exclusion of any individual SNP did not alter the inferred causal relationships (Fig. 3b).
Association of GM with immune cell
Previously, we identified Geminocystis and B-cell AC traits vital to PC. Then, we verified the causal role of Geminocystis on B-cell AC traits. The MR study revealed a correlation between Geminocystis and B-cell AC (OR: 0.448, 95% CI: 0.272 - 0.739, P = 0.002, Fig. 1 and Supplementary Material 6, wjon.elmerpub.com). Neither heterogeneity nor horizontal pleiotropy was observed, and the leave-one-out sensitivity analysis showed that no SNP notably modified the stable association (Supplementary Material 3, wjon.elmerpub.com and Fig. 3c).
Mediation analysis
We conducted an analysis to determine if B-cell AC acts as an intermediary in the pathway linking Geminocystis to PC. We found that higher levels of Geminocystis were associated with descending levels of B-cell AC, and this reduction was subsequently linked to a higher probability of developing PC. As shown in Table 1, our study showed that B-cell AC accounted for 15.32% of the increased risk of PC associated with Geminocystis.
 Click to view | Table 1. Mediation Effect of Geminocystis on PC via B-Cell Absolute Count in TBNK Panel |
| Discussion | ▴Top |
This research sought to demonstrate the potential causal relationship between GM and PC. We deployed MR analysis to evaluate the correlation between GM and PC, as evidenced by existing GWAS data, and to determine whether immune cells mediate the causal relationship between these factors. Our results indicated that Geminocystis elevated the risk of PC by suppressing the functionality of immune cells, and 15.321% of this effect was mediated through B-cell AC trait of TBNK panel.
The GM, a diverse assembly of archaea, fungi, bacteria, viruses and phages, resides within the digestive tract. This intricate community is fundamental to the proper functioning of the digestive system and the overall health of the individual. Previous studies have emphasized the importance of GM in the pathophysiological processes of various gastrointestinal tumors, including PC. An MR study indicated that the phylum Verrucomicrobia was associated with a reduced risk of intrahepatic cholangiocarcinoma, whereas the Bacillales is associated with an increased risk of PC [20]. Another research showed that Senegalimassilia acts as a safeguard against PC, while Odoribacter, Ruminiclostridium 9, Ruminococcaceae (UCG011), and Streptococcus are linked to an increased likelihood of the disease’s onset [21].
The GM may have an effect on regulating the host’s immune system and maintaining a stable internal environment [22]. The GM can promote the development of the gut-associated lymphoid tissue (GALT), and regulate the function of the mucosal barrier and the secretion of cytokines. Furthermore, the GM metabolites have the ability to enter the circulation and diffuse to different organs via paracellular transit or by being transported in conjunction with chylomicrons. They are involved in supervising and directing the development and function of various immune cells, including innate and adaptive immunity [23]. SCFAs have been identified to stimulate the maturation of naive CD4 T cells into Th1 cells by triggering the mTOR-S6K signaling pathway [23, 24] SCFAs can directly upregulate acetyl-CoA levels of B cells, mitochondrial energy output, fatty acid synthesis, and mTOR-regulated glycolysis, contributing to the differentiation of plasma cells and the production of antibodies [23, 25]. Other research revealed that deoxycholic acid produced by bacteria with 7α-dehydroxylating activity, along with secondary bile acids, is considered to increase the risk of PC [26]. Similarly, butyrate, a metabolite of gut bacteria, can participate in the pathophysiological process of PC and its response rate to treatment in vitro and in vivo experiment. Additionally, it can mitigate some of the damage associated with the cancer itself or caused by chemotherapy [27].
The tumor microenvironment in PC is highly immunosuppressive, containing numerous immune cells, including T cells, macrophages, DCs, and B cells [28]. The role of B cells in the tumor microenvironment is ambivalent [29]. They can exert antitumor effects by producing antibodies, facilitating antigen presentation, and supporting T-cell responses. However, they can also promote tumor progression by secreting inhibitory cytokines that suppress immune reactions. Studies have proved that B regulatory cells can release cytokines like interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β), which will elicit suppression by inhibiting the function of effector T cells and NK cells and promoting growth and activity of regulatory T cells [30]. On the other hand, B cells can serve as antigen-presenting cells or produce antibodies. In the humoral immune response of the host against tumors, the presence of B cells and their subsets within the cancer tissue has certain advantages [31, 32]. Overall, B cells play a complex and diverse role in the occurrence and progression of PC, with different types of subgroups promoting or inhibiting tumor growth through various mechanisms.
About Geminocystis, it is a new genus that was isolated from Synechocystis by Korelusova et al in 2009, and it mainly includes two species: Geminocystis herdmanii and Geminocystis papuanica [33]. Another potential new species has been identified and named Geminocystis urbisnovae [34]. Studies have suggested that Geminocystis is a sister group to the Cyanobacteria in terms of phylogenetic evolution. One study indicated that oral Cyanobacteria may be an independent risk factor for hepatocellular carcinoma [35]. However, research also suggests that multiple cyanobacteria synthesize microcystin-LR, which exhibits cytostatic properties, rendering them potential candidates for further drug design [36]. In a cross-sectional study, there was a negative correlation between Geminocystis and M0 macrophages in the alveolar lavage fluid of smokers [37]. Another study demonstrated that microbiomes in mice can modulate tumor-associated macrophages through Toll-like receptor signaling, thereby promoting immune tolerance [9]. In the tumor microenvironment of PC, memory B cells produce antibodies against tumor antigens, which activate NK cells and macrophage functions [30]. However, it has also been found that lipopolysaccharides (LPS) secreted by microbes can stimulate the secretion of the immunosuppressive factor IL-35 by some B regulatory cells consequently leading to tumor proliferation [38]. To date, no studies have been conducted on the effects of the Geminocystis genus on human health, and this study is the first study to identify a correlation between this genus and PC. However, the exact mechanisms require further investigation in subsequent research.
We confessed several limitations in our study. Firstly, the majority of the study population is of European heritage, which may influence the validity and generalizability of our findings. Secondly, constraints within the database requiring analysis of GM at the genus level may reduce the level of detail in the results. As a result, certain potential discoveries might be overlooked. Additionally, our threshold for GM IVs is set at P < 5 × 10-5, which aims to achieve more comprehensive results. However, this may lead to false positives or miss important genetic variations associated with immune cell traits and PC. This adjustment may introduce some bias into our conclusions. Meanwhile, there may be other mediators that require further investigation. Finally, it is important to note that the findings of this research have not yet been confirmed through external validation within a clinical setting, which is a limitation to be recognized.
Conclusion
Our study clarified the causal relationships between GM, immune cells, and PC. Geminocystis has been identified to raise the risk of PC, with this effect being mediated by the B-cell AC in the TBNK panel as measured. This study provides insight into the causal contributions of GM in PC, which is beneficial to the prevention and treatment of PC.
| Supplementary Material | ▴Top |
Suppl 1. STROBE-MR checklist of the study.
Suppl 2. The instruments of gut microbiota/immune cells/pancreatic cancer.
Suppl 3. The bidirectional two-sample Mendelian randomization (MR) analysis results between gut microbiota and pancreatic cancer.
Suppl 4. Pleiotropy and heterogeneity test for the Mendelian randomization (MR) analysis results.
Suppl 5. Mendelian randomization (MR) analysis on the causal effect between immune cells and pancreatic cancer.
Acknowledgments
For the data used in the present study, we gratefully acknowledge the GWAS Catalog (https://www.ebi.ac.uk/gwas/) and FinnGen (https://www.finngen.fi/en/access_results).
Financial Disclosure
This study was supported by the National Natural Science Foundation of China Project (82203086) and the Guangdong Basic and Applied Basic Research Foundation Project (2023A1515010916).
Conflict of Interest
The authors have declared that no competing interest exists.
Informed Consent
Not applicable.
Author Contributions
Wang, Zhang, and Pan designed the study. Wang and Yu collected and analyzed the data. Wang, Zhang and Pan drafted the manuscript. Li and Lei supervised the study and revised the manuscript. All authors discussed the results and contributed to the final manuscript.
Data Availability
The GWAS summary dataset for PC is available through the FinnGen Consortium R10 release (https://r10.finngen.fi/). The GM datasets are derived from the GWAS Catalog (https://www.ebi.ac.uk/gwas/, from GCST90032172 to GCST90032644). The data on immune traits are available through the GWAS Catalog (from GCST0001391 to GCST0002121).
| References | ▴Top |
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33.
doi pubmed - Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10(10):607-620.
doi pubmed - Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15(6):333-348.
doi pubmed - Foley K, Kim V, Jaffee E, Zheng L. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016;381(1):244-251.
doi pubmed - Watanabe K, Luo Y, Da T, Guedan S, Ruella M, Scholler J, Keith B, et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight. 2018;3(7):e99573.
doi pubmed - Dong Y, Zhang K, Wei J, Ding Y, Wang X, Hou H, Wu J, et al. Gut microbiota-derived short-chain fatty acids regulate gastrointestinal tumor immunity: a novel therapeutic strategy? Front Immunol. 2023;14:1158200.
doi pubmed - Pan P, Zhu Z, Oshima K, Aldakkak M, Tsai S, Huang YW, Dong W, et al. Black raspberries suppress pancreatic cancer through modulation of NKp46(+), CD8(+), and CD11b(+) immune cells. Food Front. 2020;1(1):70-82.
doi pubmed - Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925-1926.
doi pubmed - Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8(4):403-416.
doi pubmed - Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377-389.
doi pubmed - Qin Y, Havulinna AS, Liu Y, Jousilahti P, Ritchie SC, Tokolyi A, Sanders JG, et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54(2):134-142.
doi pubmed - Orru V, Steri M, Sidore C, Marongiu M, Serra V, Olla S, Sole G, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52(10):1036-1045.
doi pubmed - Chen J, Yu X, Wu X, Chai K, Wang S. Causal relationships between gut microbiota, immune cell, and Non-small cell lung cancer: a two-step, two-sample Mendelian randomization study. J Cancer. 2024;15(7):1890-1897.
doi pubmed - Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, Davey Smith G, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21(3):223-242.
doi pubmed - Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707-713.
doi pubmed - Pu B, Gu P, Zheng C, Ma L, Zheng X, Zeng Z. Self-reported and genetically predicted effects of coffee intake on rheumatoid arthritis: Epidemiological studies and Mendelian randomization analysis. Front Nutr. 2022;9:926190.
doi pubmed - Yuan S, Kar S, Vithayathil M, Carter P, Mason AM, Burgess S, Larsson SC. Causal associations of thyroid function and dysfunction with overall, breast and thyroid cancer: A two-sample Mendelian randomization study. Int J Cancer. 2020;147(7):1895-1903.
doi pubmed - Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525.
doi pubmed - http://www.r-project.org.
- Su Q, Jin C, Bo Z, Yang Y, Wang J, Wang J, Zhou J, et al. Association between gut microbiota and gastrointestinal cancer: a two-sample bi-directional Mendelian randomization study. Front Microbiol. 2023;14:1181328.
doi pubmed - Jiang Z, Mou Y, Wang H, Li L, Jin T, Wang H, Liu M, et al. Causal effect between gut microbiota and pancreatic cancer: a two-sample Mendelian randomization study. BMC Cancer. 2023;23(1):1091.
doi pubmed - Wang J, Zhu N, Su X, Gao Y, Yang R. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells. 2023;12(5):793.
doi pubmed - Yang W, Cong Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol Immunol. 2021;18(4):866-877.
doi pubmed - Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8(1):80-93.
doi pubmed - Kim M, Qie Y, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016;20(2):202-214.
doi pubmed - Cao H, Xu M, Dong W, Deng B, Wang S, Zhang Y, Wang S, et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int J Cancer. 2017;140(11):2545-2556.
doi pubmed - Panebianco C, Villani A, Pisati F, Orsenigo F, Ulaszewska M, Latiano TP, Potenza A, et al. Butyrate, a postbiotic of intestinal bacteria, affects pancreatic cancer and gemcitabine response in in vitro and in vivo models. Biomed Pharmacother. 2022;151:113163.
doi pubmed - Huber M, Brehm CU, Gress TM, Buchholz M, Alashkar Alhamwe B, von Strandmann EP, Slater EP, et al. The immune microenvironment in pancreatic cancer. Int J Mol Sci. 2020;21(19):7307.
doi pubmed - Gupta SL, Khan N, Basu S, Soni V. B-cell-based immunotherapy: a promising new alternative. Vaccines (Basel). 2022;10(6):879.
doi pubmed - Delvecchio FR, Goulart MR, Fincham REA, Bombadieri M, Kocher HM. B cells in pancreatic cancer stroma. World J Gastroenterol. 2022;28(11):1088-1101.
doi pubmed - Tarlinton D. B cells still front and centre in immunology. Nat Rev Immunol. 2019;19(2):85-86.
doi pubmed - Delvecchio FR, Fincham REA, Spear S, Clear A, Roy-Luzarraga M, Balkwill FR, Gribben JG, et al. Pancreatic cancer chemotherapy is potentiated by induction of tertiary lymphoid structures in mice. Cell Mol Gastroenterol Hepatol. 2021;12(5):1543-1565.
doi pubmed - Korelusova J, Kas tovsky J, Komarek J. Heterogeneity of the cyanobacterial genus synechocystis and description of a new genus, Geminocystis(1). J Phycol. 2009;45(4):928-937.
doi pubmed - Goulart MR, Stasinos K, Fincham REA, Delvecchio FR, Kocher HM. T cells in pancreatic cancer stroma. World J Gastroenterol. 2021;27(46):7956-7968.
doi pubmed - Polyakova E, Averina S, Pinevich AJA. Geminocystis urbisnovae sp. nov.(Chroococcales, Cyanobacteria): polyphasic description complemented with a survey of the family Geminocystaceae. 2023;38:93-110.
- Kounnis V, Chondrogiannis G, Mantzaris MD, Tzakos AG, Fokas D, Papanikolaou NA, Galani V, et al. Microcystin LR shows cytotoxic activity against pancreatic cancer cells expressing the membrane OATP1B1 and OATP1B3 transporters. Anticancer Res. 2015;35(11):5857-5865.
pubmed - Shields PG, Ying KL, Brasky TM, Freudenheim JL, Li Z, McElroy JP, Reisinger SA, et al. A pilot cross-sectional study of immunological and microbiome profiling reveals distinct inflammatory profiles for smokers, electronic cigarette users, and never-smokers. Microorganisms. 2023;11(6):1405.
doi pubmed - Michaud D, Mirlekar B, Steward C, Bishop G, Pylayeva-Gupta Y. B cell receptor signaling and protein kinase D2 support regulatory B cell function in pancreatic cancer. Front Immunol. 2021;12:745873.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.