| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 15, Number 6, December 2024, pages 853-870
Updates on Breast Reconstruction: Surgical Techniques, Challenges, and Future Directions
Ryohei Katsuragia, b, c, m, Cemile Nurdan Ozturkd, Kohei Chidab, e, Gabriella Kim Mannb, Arya Mariam Royf, Kenichi Hakamadae, Kazuaki Takabeb, g, h, i, j, k, l, Toshihiko Satakea
aDepartment of Plastic, Reconstructive and Aesthetic Surgery, University of Toyama, Toyama 930-0152, Japan
bDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, USA
cDepartment of Breast Surgery, Nakagami Hospital, Okinawa 904-2142, Japan
dDepartment of Head and Neck/Plastic and Reconstructive Surgery, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, USA
eDepartment of Gastroenterological Surgery, Hirosaki University Graduate School of Medicine, Hirosaki 036-8562, Japan
fDepartment of Hematology and Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, USA
gDepartment of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine, Yokohama, Kanagawa 236-0004, Japan
hDepartment of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, The State University of New York, Buffalo, NY 14263, USA
iDepartment of Breast Surgery and Oncology, Tokyo Medical University, Tokyo 160-8402, Japan
jDivision of Digestive and General Surgery, Niigata University Graduate School of Medical and Dental Sciences, Niigata 951-8510, Japan
kDepartment of Breast Surgery, Fukushima Medical University School of Medicine, Fukushima 960-1295, Japan
lDepartment of Breast Surgery, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, USA
mCorresponding Author: Ryohei Katsuragi, Department of Plastic, Reconstructive and Aesthetic Surgery, University of Toyama, Toyama 930-0152, Japan
Manuscript submitted August 2, 2024, accepted September 13, 2024, published online October 6, 2024
Short title: Updates on Breast Reconstruction
doi: https://doi.org/10.14740/wjon1935
- Abstract
- Introduction
- Implant-Based Reconstruction
- Autologous Breast Reconstruction
- Patient Reported Outcomes (PROs)
- Breast Reconstruction With Fat Grafting (FG)
- Future Directions
- Conclusions
- References
| Abstract | ▴Top |
The increasing global incidence of breast cancer underscores the significance of breast reconstruction in enhancing patients’ quality of life. Breast reconstruction primarily falls into two categories: implant-based techniques and autologous tissue transfers. In this study, we present a comprehensive review of various aspects of implant-based reconstruction, including different types of implants, surgical techniques, and their respective advantages and disadvantages. For autologous breast reconstruction, we classified flaps and optimal harvest sites and provided detailed insights into the characteristics, benefits, and potential complications associated with each flap type. In addition, this review explores the emerging role of fat grafting, which has received increasing attention in recent years. Despite advancements, there remains substantial scope for further improvements in breast reconstruction, emphasizing not only aesthetic outcomes, but also a reduction in complications and postoperative recovery. By offering a comprehensive overview of the historical evolution, current landscape, and future prospects of breast reconstruction, this review aims to provide readers with a comprehensive understanding of breast cancer management strategies.
Keywords: Breast reconstruction; Breast implant; Autologous breast reconstruction; Fat grafting
| Introduction | ▴Top |
Breast cancer continues to represent a significant global health challenge, imposing both medical and psychological burdens on millions of individuals annually. According to the most recent World Cancer Burden Statistics Report by the World Health Organization (WHO) Institute for Research on Cancer, approximately 2.3 million new cases of breast cancer were reported in 2022, accounting for 11.6% of all new cancer cases, making it the most prevalent malignant disease globally [1].
Total mastectomy is a common treatment for breast cancer, but it often leaves individuals grappling with the substantial psychological impact of breast loss. The fundamental goal of breast reconstruction is to achieve optimal cosmetic outcomes following breast cancer surgery. In addition, there is increased recognition of the significance of patient-reported outcomes (PROs), emphasizing the importance of reducing complications and enhancing postoperative quality of life [2]. Immediate breast reconstruction has proven beneficial by mitigating psychological distress, improving overall quality of life [3], and reducing healthcare costs compared to delayed breast reconstruction [4]. Furthermore, it has demonstrated oncological safety, with no increased risk of breast cancer recurrence or distant metastasis [5]. This ongoing evolution underscores the substantial progress in the field of breast reconstruction, reflecting a steadfast commitment to improving patient experiences and outcomes.
This review aims to provide a comprehensive summary of contemporary breast reconstruction techniques, encompassing their historical evolution, prevalent types, associated complications, as well as emerging innovative approaches that have garnered significant attention recently.
| Implant-Based Reconstruction | ▴Top |
Breast reconstruction methods can be classified into two main categories: implant-based reconstruction and autologous tissue transfer (Table 1) [6-12]. The most significant advantage of implant-based reconstruction is that it does not require a donor site and is less invasive than autologous reconstruction. Implant-based reconstruction remains the most commonly employed technique, accounting for 80% of all breast reconstruction procedures in the USA [13]. Breast implants date back to the 1950s, with various materials used for breast augmentation and reconstruction, including ivory, glass, sponge, polyurethane, and polytetrafluoroethylene. However, these materials often resulted in unfavorable local tissue reactions, causing the breasts to stiffen and deform over time with poor outcomes [14]. The development of silicone implants in the 1960s marked a significant advancement and the onset of the modern era of breast augmentation and reconstruction.
 Click to view | Table 1. Patient Selection Criteria for Breast Reconstruction Methods |
Types of implants
Various types of breast implants have been used for breast reconstruction. Implants can be classified into silicone- or saline-filled based on their contents, and smooth or textured according to their surface. Silicone implants are currently the most commonly used type of implant. The original implant had a smooth shell, while textured surface implants were later developed to reduce migration and capsular contracture. However, in the last decade, there has been a shift back to smooth implant use due to increased concerns regarding the development of lymphoma around textured devices.
In 1962, Cronin et al first reported the use of silicone gel breast implants [15]. Three years later, the first inflatable saline-filled breast implant became available [16]. Improvements continued to be made over time; however, in the 1980s, patients began suing implant manufacturers, claiming that silicone implants caused various complications, including autoimmune diseases and breast cancer, which were not present prior to surgery. In 1992, the US Food and Drug Administration (FDA) banned the use of silicone implants, citing insufficient evidence supporting their safety and effectiveness. Consequently, saline-filled implants became the only available treatment option in the USA [17]. However, after thorough evaluation, the claim that breast implants were associated with breast cancer and connective tissue disease was denied [18], and the FDA lifted the moratorium in 2006. After their reintroduction to the USA and Canadian markets, silicone gel implants became the prosthesis of choice.
Compared to saline implants, silicone implants offer a softer and more natural feel and appearance and are less prone to rupture or degradation [19]. This is especially true in patients with thin skin or a slender physique, where silicone implants are more likely to achieve a natural look. However, if safety in the event of rupture or cost is a priority, saline implants may be preferred. A survey by the American Society of Plastic Surgeons (ASPS) reported that 82% of surgeons preferred silicone implants, with 33% using them exclusively [20].
In the 1960s, the surfaces of early implants were smooth, but they exhibited a high rate of capsular contracture (10.6-24.6%), representing a major disadvantage [16, 21]. It became clear that surface properties play a role in capsular contracture, leading to the development of textured implants in the 1970s [14]. Textured-surface silicone implants, including Mentor’s Siltex imprinted implants (Mentor Worldwide LLC, Irvine, CA) and Allergan’s Biocell salt-loss devices (Allergan, Irvine, CA), were introduced in the late 1980s, over a decade after the first documented use of polyurethane textured implants [22, 23].
The incidence of capsular contracture decreased with the advent of textured implants [6]. Specifically, tear-drop-shaped implants became popular as the textured shell prevented implant rotation by adhering to the surrounding tissues. However, continuous use of textured implant types has been linked to inflammation, immune reactions, and chronic infections [24]. Furthermore, in the 2010s, emerging evidence suggested that patients with textured implants might be at risk of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) [25]. This led to the recall of the Allergan Biocell-textured implant from the USA and the global markets in 2019. Owing to these disadvantages, smooth-shell implants are increasingly being used.
Anatomical plane of reconstruction
Breast reconstruction can be performed in two anatomical planes for expander and implant insertion: subpectoral and prepectoral (Fig. 1, Table 2) [6, 16, 19, 21, 25-32]. Although expanders or implants were initially inserted directly into the prepectoral space, this method was abandoned because of the high rate of complications [33]. For a period, subpectoral placement became the mainstay of reconstruction. However, with the advent of acellular dermal matrices (ADMs), a biomaterial derived from processed human or animal skin designed to remove cells while preserving the extracellular matrix, and advancements in total mastectomy techniques, prepectoral reconstruction is becoming increasingly popular.
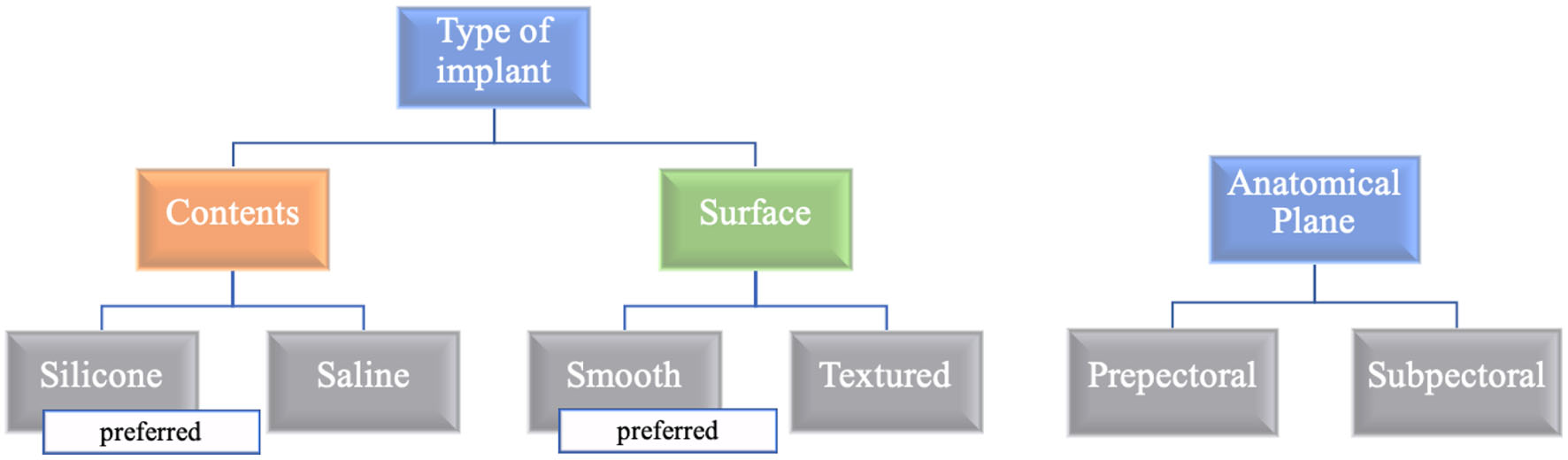 Click for large image | Figure 1. Types of implants and anatomical planes of reconstruction. This figure shows a flowchart of implant types and anatomical planes. In terms of contents, silicone implants are preferred for their softness, while smooth implants are favored due to concerns about breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). For the anatomical plane, the prepectoral option is chosen when the mastectomy flap has sufficient thickness. The use of acellular dermal matrix (ADM) is required in the prepectoral plane. |
 Click to view | Table 2. Comparison of Different Methods of Implant-Based Reconstruction |
In the subpectoral method, a tissue expander (TE) is traditionally inserted into the subpectoral plane, and the lateral part is covered by the serratus anterior muscle or fascia. After several weeks of percutaneous saline infusion and gradual expansion, the TE is replaced with an implant (Fig. 2). Since the early 1980s, this approach has been the preferred surgical technique [33]. However, the disadvantages of subpectoral reconstruction include animation deformity, muscle tightness, increased discomfort, and lateral malpositioning of the implant due to continuous pectoralis movement. ADM was first used in breast reconstruction by Breuing et al in 2005 as a sling to support the lower pole of the pectoralis major during subpectoral breast reconstruction (Fig. 3) [34]. The pectoralis major muscle is detached from the chest wall, and its inferior edge is sutured to the ADM, which functions as an extension of the muscle to form a continuous plane. This technique eliminates the need to expand the pectoralis major muscle, allowing for implant insertion to be completed in a single operation, without multiple surgeries and outpatient visits. ADM use also results in significantly less capsular contracture than muscle-only implant coverage.
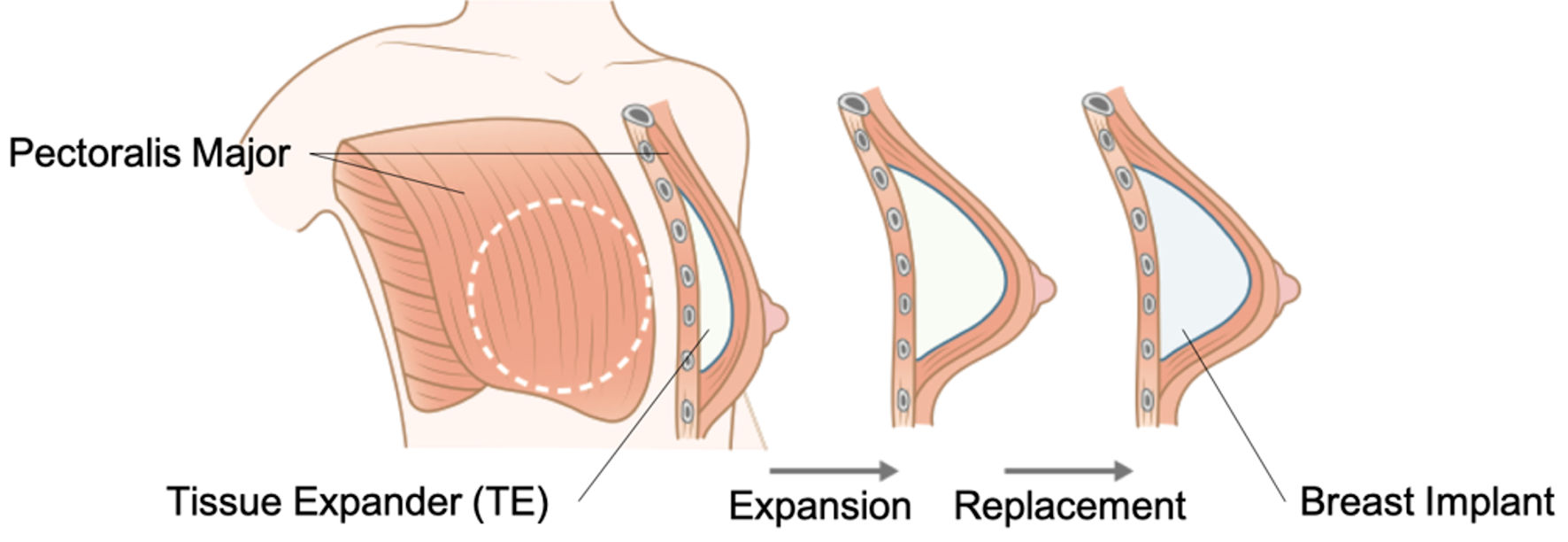 Click for large image | Figure 2. Subpectoral reconstruction. The tissue expander (TE) is inserted into the subpectoral plane. After several months of percutaneous saline infusion and gradual expansion, the TE will be replaced with the breast implant. |
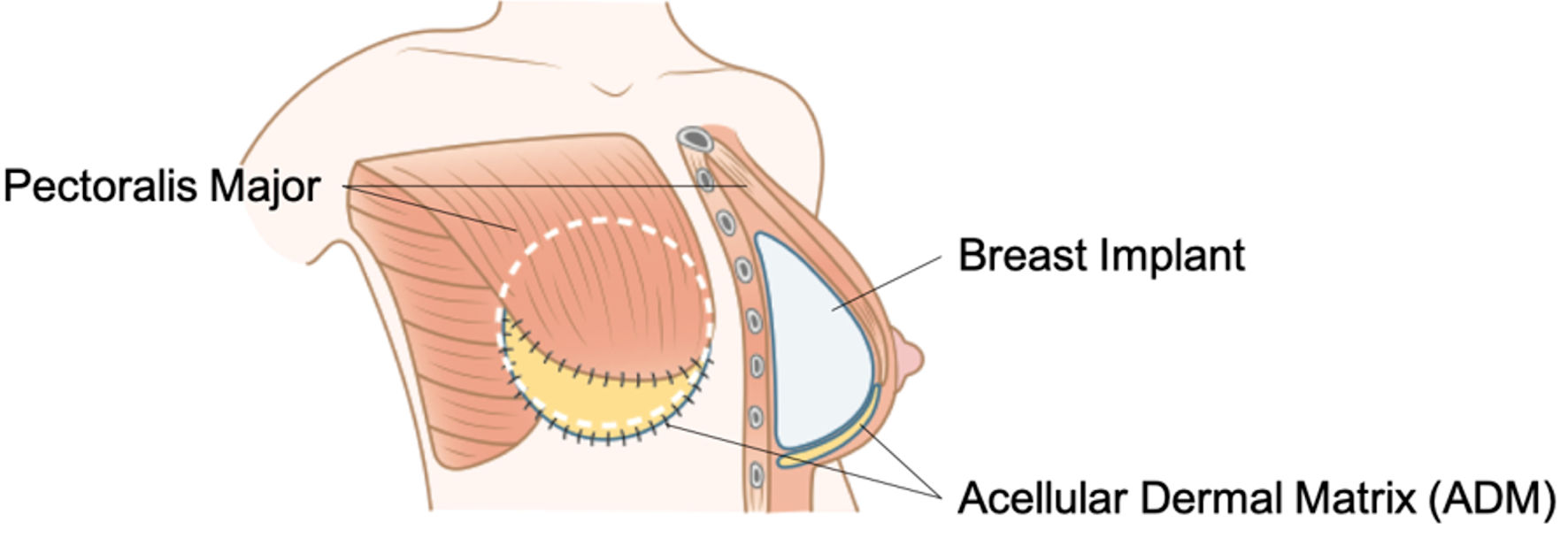 Click for large image | Figure 3. Subpectoral reconstruction with acellular dermal matrix (ADM). The pectoralis major muscle is detached inferiorly, and then sutured to the ADM to create a single plane. This eliminates the need for expanding the pectoralis major muscle using a tissue expander (TE), allowing for the implant to be inserted in a single phase. |
A less invasive prepectoral method in which the TE or implant is covered with an ADM and inserted at the anterior aspect of the pectoralis major muscle is becoming increasingly popular (Fig. 4). There are two types of prepectoral methods using ADM: the wrap-around method, which covers the entire implant, and the anterior cover method, which covers only the anterior portion [35]. The prepectoral method offers multiple advantages, including reduced animation deformity, less chronic pain, easier reconstruction of ptotic breasts, and decreased capsular contracture when ADM is used [26-28]. Both the prepectoral and subpectoral methods have similar complication rates, indicating comparable safety [26, 36, 37]. However, the prepectoral approach requires adequate mastectomy skin flap thickness and optimal blood flow, making it unsuitable for skin defects. Patients with thinner skin envelopes are also more prone to implant visibility and rippling [29].
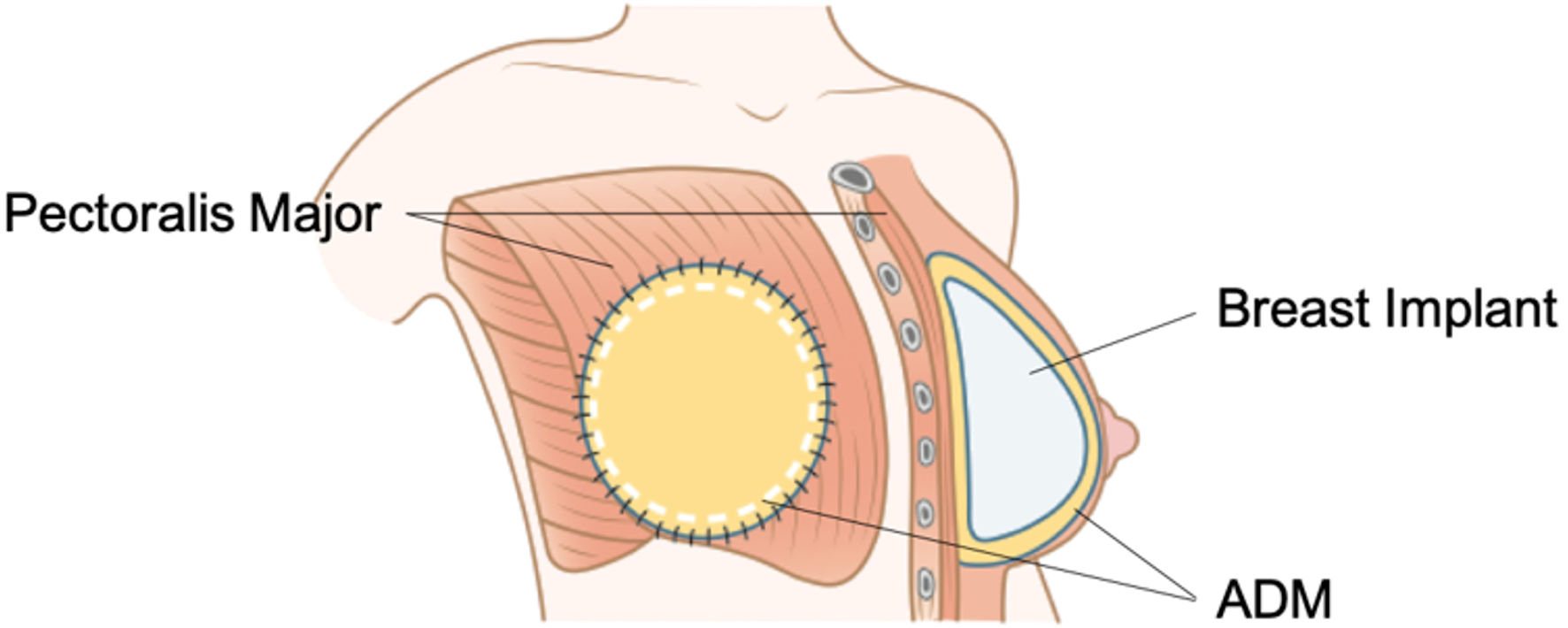 Click for large image | Figure 4. Prepectoral reconstruction. The breast implant is entirely covered with acellular dermal matrix (ADM) and inserted at the anterior aspect of pectoralis major muscle. This method is less invasive and becoming more widely used. |
ADM has improved both the prepectoral and subpectoral methods. In a survey by the ASPS, 48.4% of respondents employed the prepectoral procedure, but concerns regarding the high cost and off-label ADM use persisted [38]. A systematic review by Daar et al [39] illustrated a decrease in two-stage reconstruction post-nipple-sparing mastectomy (NSM), with a consistent increase in direct-to-implant reconstructions over the past decade.
Complications associated with implant-based reconstruction
Capsular contracture
When the body identifies a foreign substance, it instinctively responds by forming a barrier of scar tissue to encapsulate it. Similarly, a capsule of scar tissue forms around the implant to maintain its position and prevent shifting. However, in some patients, capsular contracture can occur, where the scar tissue capsule becomes abnormally hard and contracts around the implant, leading to pain and implant deformation in severe cases. The incidence of capsular contracture ranges from 5.7% to 14.5%, and its frequency increases over time from the date of surgery [6, 40].
The risk factors associated with capsular contracture include radiation therapy (RT), biofilm contamination, and surgical site infection (SSI) [41]; however, the exact pathogenesis of capsular contracture remains unclear. The incidence of capsular contracture can be reduced with the prophylactic administration of antibiotics and use of textured implants and ADMs [7], while the risk of capsular contracture is lower, and the duration of expansion is shorter when TEs are inserted [6].
SSI
SSI after implant-based breast reconstruction adversely affects surgical outcomes. The infection rates in implant reconstruction range from 3.2% to 14.8% [26, 40, 42] with approximately half of cases occurring within the first 60 days post-surgery [40]. The high rate of infection is attributed to the presence of bacteria in the mammary gland, flap ischemia after mastectomy, lymph node sampling or dissection, increased dead space, and foreign body placement [43, 44].
Common causative organisms include Staphylococcus epidermidis, Staphylococcus aureus, Escherichia, Pseudomonas, Propionibacterium, and Corynebacterium [8, 45]. The initial management of SSI involves antibiotics; however, explantation may be indicated for cases unresponsive to treatment. Recent studies have shown no benefit of extended oral antimicrobial prophylaxis in reducing SSIs [46].
Breast implant-related malignancies
BIA-ALCL is a type of T-cell non-Hodgkin lymphoma that occurs in patients with breast implants and arises from the capsular tissue that forms around the implant. The association between breast implantation and ALCL was first reported in 1997 [47] and recognized as a risk factor by the FDA in 2011. The reported incidence was approximately one in 2,207 to one in 3,345, with an onset 7 - 9 years after implant insertion [48]. Textured-type implants have been reported as a higher risk factor compared to smooth-type implants [48]. Late swelling (seroma) and mass formation are signs of BIA-ALCL that warrant fluid or capsule sampling for diagnosis. Removal of the implant with its entire capsule is the optimal surgical treatment, with chemotherapy or radiotherapy deemed unnecessary for patients with early-stage disease given its localized lesion with slow course and good prognosis.
Breast implant-associated squamous cell carcinoma (BIA-SCC) is another malignancy that can develop around breast implants and within the implant capsule. As of March 8, 2023, 19 cases have been reported [49]. Given the limited data, the lifetime risk and risk factors for BIA-SCC development remain unknown; however, it is highly aggressive and has a poor prognosis.
Implant rupture
A 10-year prospective study [6] reported an implant rupture frequency detected on magnetic resonance imaging (MRI) of 12.4%, with all ruptures occurring within the capsule and no reports of extracapsular rupture or gel migration. The FDA recommends initial MRI/ultrasound monitoring at 5 years post-implantation and every 2 - 3 years thereafter [9].
Breast implant illness (BII)
BII is characterized by various self-reported disabling and distressing physical and psychological symptoms, such as fatigue, joint pain, brain fog, muscle pain, and autoimmune disorders [50], which have gained attention, particularly due to social media. Although the mechanism of BII symptoms remains unclear, it is likely multifactorial and psychosomatic. Currently, it is not possible to confirm whether BII is a connective tissue disorder; however, it is important to scientifically establish and rule out other underlying causes of these symptoms. Most patients with BII request implant removal with total capsulectomy, and some report relief of symptoms after the procedure.
| Autologous Breast Reconstruction | ▴Top |
Autologous breast reconstruction is a surgical procedure that utilizes tissues from other parts of the patient’s body to reconstruct the breast, and offers several advantages. Unlike implants, which require follow-up to check for implant rupture or malignant lymphoma development, autologous reconstruction requires minimal maintenance. Furthermore, autologous reconstruction adapts naturally over time, undergoing similar age-related changes to those of natural breasts (e.g., sagging), eliminating the need for replacement and avoiding the risk of complications associated with implants entirely.
The history of autologous breast reconstruction (Table 3) [51-64] dates back to 1887 when tissues from the contralateral breast were used [51]. In 1906, Tansini [52, 65] introduced the use of a latissimus dorsi myocutaneous (LDMC) flap from the back. The 1970s saw development of the transverse rectus abdominis myocutaneous (TRAM) flap using abdominal tissue [53]. The deep inferior epigastric artery perforator (DIEP) flap [54], a refined version of the TRAM flap with less donor site morbidity, was utilized in the 1980s. Advancements in microsurgery in the 2000s further expanded the options available, including the gluteal artery perforator (GAP) flap [55] and profunda artery perforator (PAP) flap [56].
 Click to view | Table 3. History of Autologous Breast Reconstruction |
Understanding the differences between the flap types is crucial (Table 4) [54-56, 58, 62, 66-70]. Myocutaneous flaps, such as the LDMC and TRAM flaps, consist of muscle, subcutaneous fat, skin, and vessels, which are easy to elevate but result in increased donor site morbidity. These flaps can also lose volume due to muscle atrophy after reconstruction. In contrast, perforator flaps, such as the DIEP flap, preserve the muscle and minimize donor site morbidity, but require precise surgical techniques. Pedicled flaps maintain the original blood supply, facilitating reliable blood flow, but limit tissue mobility. Conversely, free flaps offer more flexibility and superior reshaping but are more complex due to the need for microvascular anastomosis with a higher risk of flap loss. Each technique has its unique advantages and challenges, necessitating careful selection based on patient-specific needs, anatomical factors, and surgeon expertise.
 Click to view | Table 4. Patient Selection Criteria, Advantages, and Disadvantages for Autologous Tissue Breast Reconstruction |
Recent advancements have focused on combining different flaps and incorporating nerves into the flaps to enhance sensory regeneration in reconstructed breasts. This not only restores the physical appearance but also contributes to regaining sensation, improving the overall quality of life of patients. The field is continually evolving with ongoing innovations aimed at maximizing the benefits of autologous breast reconstruction.
Flaps used in breast reconstruction
Various body regions serve as donor sites for flaps in breast reconstruction, with the back, lower abdomen, thighs, and buttocks representing the most prevalent sites (Fig. 5). The common flaps harvested from these regions are discussed below.
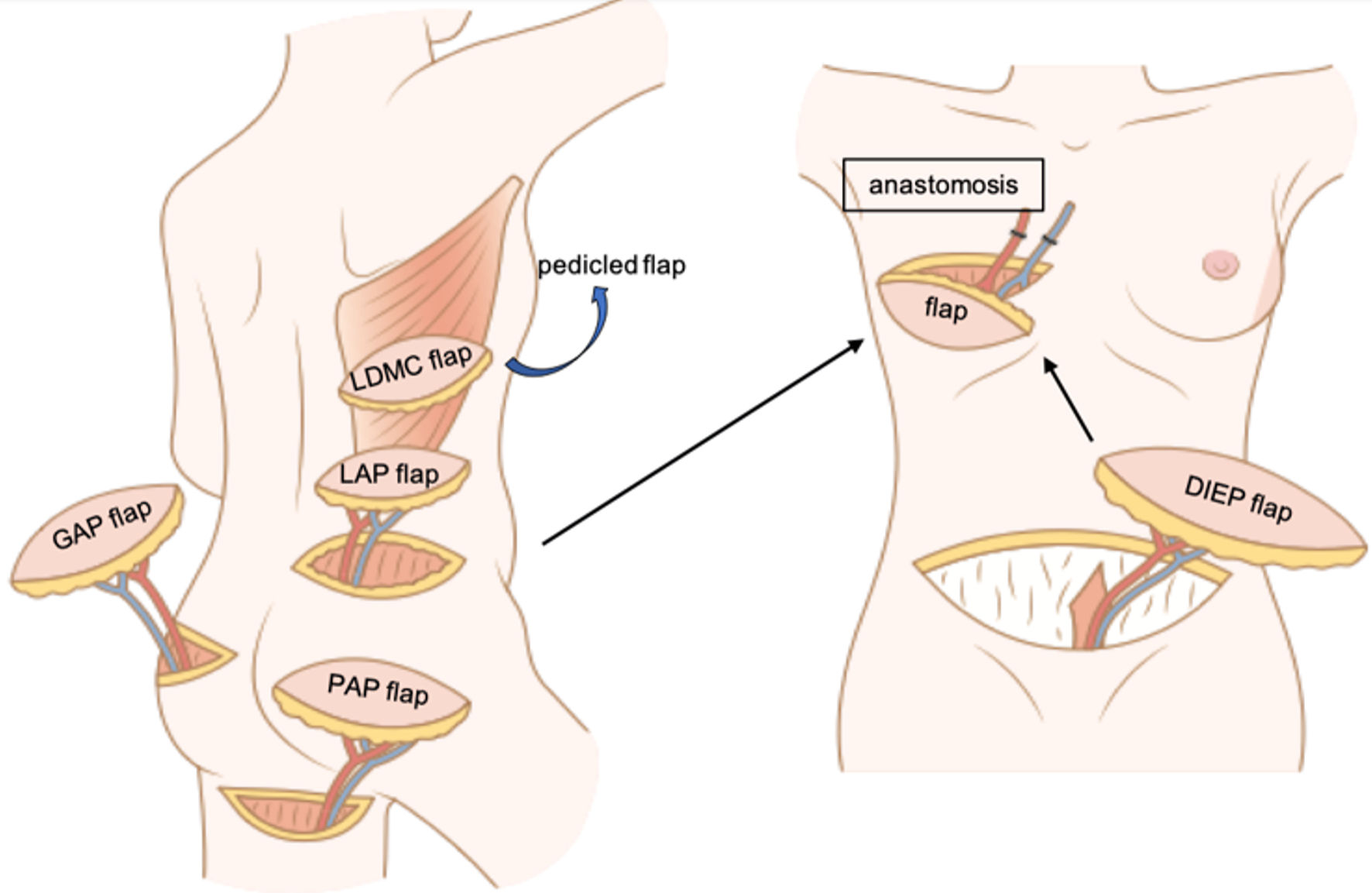 Click for large image | Figure 5. Representative examples of flaps used in autologous breast reconstruction. The LDMC flap is a pedicled myocutaneous flap, while the others are free perforator flaps that require vascular anastomoses. LDMC flap: latissimus dorsi myocutaneous flap; DIEP flap: deep inferior epigastric artery perforator flap; LAP flap: lumber artery perforator flap; PAP flap: profunda artery perforator flap; GAP flap: gluteal artery perforator flap. |
Back
1) LDMC flap
The LDMC flap, a pedicle flap based on the thoracodorsal vessels, is particularly suitable for smaller breasts, making it a popular choice in Asian populations. This flap does not require vascular anastomosis or dissection of perforating blood vessels [66].
However, one limitation is its relatively small volume. Recent innovations, such as the synergistic use of implants and adipose injections, have been effective in augmenting volume, enabling the reconstruction of larger breasts [71, 72]. Another drawback is the prominent scar remaining on the back, but advances in endoscopic harvesting of flaps without skin paddles and injection of adipose tissue into the myocutaneous flap have minimized scarring while preserving flap volume [57], allowing the LDMC flap to remain a viable and refined option.
2) Lumber artery perforator (LAP) flap
LAP flaps [58] from the lumbar region offer several advantages, including consistent perforating branch positioning, ease of elevation, and well-concealed scars beneath clothing. In addition, the procured volume has been reported to be substantial, averaging 651 g, with a soft fat texture closely resembling that of the breast [67]. However, the free LAP flap has a short vascular pedicle, averaging 4.0 cm [67], necessitating intraoperative adjustments and rendering it more of a salvage procedure, rather than a primary choice [68]. Recent innovations have demonstrated its efficacy in conjunction with a DIEP flap when increased flap dimensions are necessary [73].
Lower abdomen
1) Transverse rectus abdominis muscle flap
The abdomen is the predominant donor site for autologous breast reconstruction, primarily due to the abundance of adipose tissue commonly found in women requiring breast reconstruction [68]. The abdominal flap offers several advantages, including its soft adipose tissue, which is similar to the texture of breast tissue, facilitating the replication of the natural breast morphology. Furthermore, the ensuing abdominal contours are often aesthetically pleasing [74].
In 1982, Hartrampf et al [69] introduced a pedicled TRAM flap utilizing the superior epigastric vessels. Advancements in microsurgery have enabled the refinement and increased adoption of free TRAM flaps, which rely on the inferior epigastric vessels known for superior perfusion [75]. This progression enhances vascular stability of the TRAM flap, mitigates the risk of fat necrosis, and provides enhanced flexibility for breast mound formation [76].
DIEP flaps alleviate donor site morbidities typically associated with TRAM flaps, such as hernias and bulging [77]. Given these advantages, DIEP flaps are often preferred over TRAM flaps in contemporary practice. However, pedicled TRAM flaps remain a viable alternative in the absence of microsurgical options.
2) DIEP flap
The DIEP flap is regarded as the gold standard for breast reconstruction in institutes with microsurgical capabilities [68]. However, limitations include patients considering pregnancy, scarce subcutaneous fat in the abdomen, complex surgical scars in the abdominal region, or history of abdominoplasty.
The DIEP flap derives its blood supply from perforating branches of the deep inferior epigastric artery and vein (DIEA/V), which emanate from the external iliac artery and perforate the rectus abdominis muscle. Most perforating vessels are located around the umbilicus [78].
In unilateral reconstruction, only one DIEA/V is harvested. However, a bipedicled DIEP flap can be used to involve the contralateral DIEA/V when more extensive blood supply to the flap is desired. The DIEP flap is versatile and can cover substantial skin defects after mastectomy. Recent advancements have allowed the incorporation of the intercostal nerve in the flap, which can be coapted to the intercostal nerve in the chest. This technique has enabled not only cosmetic but also sensate reconstruction [59].
3) Superficial inferior epigastric artery (SIEA) flap
The SIEA flap, similar to the DIEP flap, provides abdominal tissue for breast reconstruction but does not necessitate incision of the abdominal fascia or rectus muscle, as its vascular supply (SIEA/V) originates from the common femoral artery. This method significantly mitigates the risk of abdominal complications, such as hernia or bulging [60]. However, the SIEA flap has several disadvantages, including a smaller pedicle diameter and shorter pedicle length than those of the DIEP flap. Additionally, patients may present with an insufficient arterial pedicle, making application of the SIEA flap unsuitable [79]. Nonetheless, although not as frequently employed as the DIEP flap, the SIEA flap remains a valuable option for selected patients and offers certain advantages over DIEP flaps, particularly in terms of reducing abdominal morbidity.
Thigh
1) PAP flap
The PAP flap, first documented by Allen et al for breast reconstruction [56], is increasingly recognized as a viable alternative to the DIEP flap. It originates from the medial thigh, can be designed transversely or longitudinally [80], and is vascularized by a perforating branch of the deep femoral artery that penetrates the adductor magnus muscle.
The advantages of the PAP flap include an inconspicuous scar at the donor site and sufficient pedicle length, averaging approximately 10 cm [81]. Additionally, it allows for muscle preservation and can be elevated during mastectomy while the patient is in prone position. However, its relatively modest weight of approximately 367 g [81] limits its suitability primarily to small-to medium-sized breast reconstructions. Nonetheless, it remains an effective option, particularly when less-noticeable scars and muscle preservation are paramount.
2) Transverse upper gracilis (TUG) flap/diagonal upper gracilis (DUG) flap
The TUG [61] and DUG [82] flaps are myocutaneous flaps sourced from the medial thigh. They can be distinguished by their orientation; the TUG flap runs along the transverse axis, whereas the DUG flap runs along the vertical axis. The predominant pedicle is the medial femoral circumflex artery, accompanied by two venae comitantes.
The TUG flap has certain disadvantages such as limited availability of skin and soft tissue, shorter pedicle length [83], and a higher risk of inducing lymphedema in the lower extremities post-harvest than that of the DUG flap [84]. Studies have demonstrated that the DUG flap tends to be a more reliable option, owing to the constraints of the TUG flap [85]. Both flaps are considered less technically demanding than the PAP flap because they do not necessitate dissection of the perforating branch. However, a significant drawback shared by both methods is the sacrifice of functional muscles, a factor that requires careful consideration during the decision-making process.
Buttocks
1) Superior gluteal artery perforator flap
Introduced in 1995 by Allen et al [55], the superior gluteal artery perforator (SGAP) flap derives its vascular supply from perforating branches of the superior gluteal artery, which emanate from the internal iliac artery. This flap offers the distinct advantage of harvesting abundant fat from the buttock area, akin to the abdomen, with inconspicuous resultant scars. However, the flap features a relatively short pedicle with an average length of 7.8 cm (range: 6 - 10.5 cm) [70], potentially necessitating the use of vein grafts. Moreover, the flap requires the patient to be in the prone position during harvesting, which necessitates intraoperative repositioning, a step that might complicate the surgical procedure. Additionally, pedicle dissection can be tedious because of the long intramuscular course.
Despite these shortcomings, the SGAP flap boasts a commendable safety record, with a reported total flap loss rate of 1% and an emergent surgical re-exploration rate of 5% [86]. This places the SGAP flap on par with other flaps, such as the DIEP and PAP flaps, in terms of safety [87, 88]. Consequently, the SGAP flap serves as a viable option for patients who require a relatively large flap and for whom an abdominal flap is not suitable due to conditions such as pregnancy or a history of abdominal surgery [89].
2) Inferior gluteal artery perforator flap
The inferior gluteal artery perforator (IGAP) flap, subsequently introduced by Allen et al [62] in 1997, serves as an alternative to the abdominal flap when deemed unfeasible, similar to the SGAP flap. The inferior gluteal artery, a terminal branch of the internal iliac artery, exits the pelvis through the sciatic foramen and is situated below the piriformis muscle. This flap poses the potential risk of exposing the sciatic nerve during the harvesting process. Reportedly, approximately 19% of patients experienced sensory disturbances postoperatively [89]. Another notable disadvantage is discomfort arising from the surgical wound, especially in the load-bearing area while in a seated position. For these reasons, the SGAP flap is often the preferred choice over the IGAP flap in clinical practice.
Complications associated with autologous breast reconstruction
SSI
The reported SSI rate after autologous tissue reconstruction was 3.6%, which was lower than that of prosthetic reconstruction [90]. This lower rate can be attributed to the robust blood flow to the surgical site facilitated by tissue transplantation. The administration of antibiotics beyond 24 h did not further decrease the infection rate [91].
Microvascular thrombosis
Microvascular thrombosis is a specific complication of free autologous breast reconstruction that poses a risk of total flap loss. According to published studies, microvascular thrombosis rates range from 1.3% to 8.9% [90, 92]. While heparin may be used for postoperative thromboprophylaxis, the efficacy of intraoperative and postoperative anticoagulant use in preventing anastomotic thrombosis is limited, and systemic administration is generally not recommended [93]. The salvage rate of flaps affected by microvascular thrombosis was reported to be 50-70% following mechanical thrombectomy, vein grafts, and thrombolytic use [94].
Donor site complications
Several complications can occur at the donor site, including seroma, contour deformity, wound dehiscence, bulge/hernia, and sensory disturbances. Wound dehiscence is particularly problematic in high-risk patients, such as those who are obese or smokers. Closed incision negative pressure therapy (ciNPT) has been shown to reduce the wound dehiscence rate from 12.9% to 4.9%, offering an alternative method to standard postoperative dressings for donor sites in breast reconstruction [95]. ciNPT also significantly decreased the incidence of SSI and length of postoperative hospital stay [96].
| Patient Reported Outcomes (PROs) | ▴Top |
The primary goal of breast reconstruction is to achieve patient satisfaction with respect to the perceptions of psychosocial sequelae, physical function, and cosmetic outcomes. Thus, the evaluation of the patient’s experience is particularly important, and measurements such as patient satisfaction and health-related quality of life are important outcomes for evaluating breast reconstruction surgery.
What are PROs?
PROs are outcomes for which clinicians can receive direct reports from patients, such as health status and quality of life related to medical care and treatments. PROs before and after treatment allow for a more comprehensive evaluation of the treatment. Especially in clinical trials, where outcomes can only be observed in terms of subjective patient impact, PROs are useful outcome measures and have attracted attention in the field of plastic surgery, where it is difficult to quantify therapeutic interventions [97].
The BREAST-Q was published in 2009 as a PRO measure of health-related quality of life and satisfaction and is widely used because of its high reliability and validity [2]. The conceptual framework includes six domains: satisfaction with breasts, overall outcome and process of care, and psychosocial, physical, and sexual well-being. The BREAST-Q showed improvements in satisfaction with breast, psychosocial, physical, and sexual well-being scores after reconstruction compared with preoperative scores, demonstrating the validity of breast reconstruction in PROs [98].
PROs of implants vs. autologous tissue
Both implant and autologous tissue reconstruction have been shown to contribute to short-term improvements in patient satisfaction [99]. However, several systematic reviews have shown that autologous tissue reconstruction is more satisfactory than implant-based reconstruction [10-12].
Recently, long-term PROs have been reported in breast reconstruction. Johnson et al [100] reported 13 years of follow-up for 1,236 patients in a multicenter study, showing that autologous tissue was associated with higher satisfaction with breast, psychosocial, and physical well-being. Autologous tissue reconstruction is characterized by increased patient satisfaction over time [101], and it is important to provide this information when choosing a reconstruction method.
PROs in different implant types
There are limited studies on PRO that compare the implant content, surface, and anatomical plane in which the implants are placed. Compared with saline implants, silicone implants are associated with enhanced patient satisfaction [102]. While physical and sexual well-being were similar between silicone and saline implant recipients, psychosocial well-being was significantly better in silicone implant recipients [30]. They also reported that patient satisfaction improved when textured implants were replaced with smooth ones, regardless of the presence or absence of preoperative symptoms [31]. Furthermore, patient satisfaction was found to be similar between the prepectoral and subpectoral approaches [32]; however, the long-term outcomes and durability of prepectoral reconstruction are yet to be studied.
PROs in autologous tissue reconstruction types
Reportedly, PROs comparisons of flaps used in breast reconstruction, and a comparison of the DIEP and TRAM flaps showed a higher satisfaction rate with the DIEP flap [103, 104]. The DIEP flap uses abdominal skin and fat and does not sacrifice muscle, which allows for a natural breast look and feel, as well as faster donor site recovery and less postoperative functional impact, which may contribute to a high level of satisfaction.
A comparison of the DIEP and latissimus dorsi (LD) flaps indicated that patients who underwent reconstruction with the DIEP flap had higher scores for breast satisfaction and physical, psychological, and sexual health than those who underwent reconstruction with the LD flap [100].
Patients also expressed high long-term satisfaction, especially regarding the breast shape and appearance. Satisfaction with the PAP flap [105, 106], LAP flap [105, 107], and GAP flap [107] were comparable to that of the DIEP flap, and because the PAP flap has a limited volume that can be harvested, and LAP and GAP have short pedicles and other procedural difficulties, the DIEP flap is the gold standard for autologous tissue reconstruction. However, these flaps are good alternatives for patients who wish to preserve their fertility or have complex abdominal surgical histories that preclude DIEP flaps.
| Breast Reconstruction With Fat Grafting (FG) | ▴Top |
FG is a surgical technique where adipose tissue is harvested from a designated donor site, commonly the abdomen, thighs, or buttocks, and subsequently reinjected into the recipient site. This procedure, first introduced in 1893 by Gustav Neuber for breast reconstruction [108], has undergone substantial advancements in refining the fat purification and injection techniques, elevating its precision and efficacy.
In contemporary plastic surgery, FG has garnered significant acclaim, particularly for breast reconstruction owing to its ability to create a natural and anatomically coherent breast contour, thereby aligning with the aesthetic aspirations of patients seeking simultaneous fat reduction. Traditionally, FG was used as a revision procedure following breast reconstruction with implants or autologous tissue. However, recent innovations have facilitated the emergence of hybrid surgical approaches and the use of FG as a standard procedure for breast reconstruction.
FG for breast reconstruction is particularly beneficial for patients with adequate fat reserves and minimal to no history of RT, as these factors improve fat graft survival [109]. It is ideal for patients requiring small-to-moderate breast reconstruction and can also be utilized as a revision technique to refine breast shape and symmetry following other reconstructive surgeries.
The FG procedure is typically performed under general or local anesthesia with sedation and involves making a small incision at the donor site and injecting a solution containing adrenaline to minimize bleeding. Careful consideration of graft size is important, as larger grafts may lead to higher rates of complications, such as liquidation, necrosis, and cyst formation, while grafts that are too small can result in fat reabsorption [110]. Purified fat should be divided into small portions and injected radially in multiple directions and layers using a cannula with a 2 mm caliber blunt tip for successful fat grafting [111].
Hybrid reconstruction with both FG and implants or autologous tissue
Hybrid reconstruction combines FG with implants or autologous tissue, addressing the individual limitations of each approach. This hybrid approach significantly enhances cosmetic results and reduces postoperative pain compared with standard implant-based reconstruction [112], particularly benefiting patients undergoing RT by aiding microvascular lesion repair and reducing interstitial fibrosis [113]. As previously mentioned, when implants are positioned in the prepectoral plane, the risk of implant visibility and contour deformities is higher than when positioned in the subpectoral plane [114]. However, incorporating FG improves skin thickness, vascular supply, and shape, thereby mitigating complications associated with prepectoral implant placement [113].
Hybrid reconstruction is effective when using a LDMC flap [115-117], which by itself yields a restricted tissue volume. FG integration within the LDMC flap augments the flap volume and can be performed during the same flap transfer session in select cases; however, approximately 65% of patients require additional FG, implying that the reconstruction process may need to be repeated to achieve optimal results [117].
Total breast reconstruction using FG
Total breast reconstruction using FG has emerged as a feasible technique, with significant success, extending beyond revision or hybrid reconstruction. Studies indicate that 2.4 - 4 FG procedures may be necessary for optimal aesthetic outcomes, offering the dual benefits of fat reduction and breast reconstruction, thereby contributing to an elevated quality of life for recipients [118, 119]. Various surgical methodologies have been employed, including singular or repeated injections, perioperative attachment of expansion devices to augment the recipient site [120], and injections coupled with the reverse expansion of the TE [118]. However, standardized protocols for this approach remain elusive.
Complications associated with FG
Donor site complications
Donor site complications are more likely to occur with higher liposuction volumes [121] and are influenced by surgeon experience [122]. A systematic review found that the most common donor site complications were ecchymosis (12.0%), followed by pain (5.4%), hematoma (2.6%), and skin irregularities (0.5%) [123].
Recipient site complications
In a study De Decker et al investigating 2,419 cases of recipient site complications, fat necrosis occurred in 5.3%, oil cysts/calcification formation in 8.8%, and infection in approximately 0.96% of cases [124]. Therefore, FG must be performed while ensuring meticulous techniques to minimize these complications and optimize outcomes in breast reconstruction. Although fat embolization has been reported in the buttocks [125], no cases have been reported in breast FG procedures.
| Future Directions | ▴Top |
As the demand for breast reconstruction increases, there is growing interest in less invasive techniques and efforts to minimize postoperative complications, potentially heralding a new era in breast reconstruction.
Robotic-assisted surgery
Robot-assisted surgery has been widely adopted to treat various solid tumors, including prostate, colorectal, gastric, and gynecological cancers. Toesca et al [126] pioneered their application to NSM in 2015. While conventional NSM leaves a lengthy scar, robotic NSM (RNSM) leaves only a small scar at the port insertion site. Robotic surgical systems provide high-resolution three-dimensional (3D) images, flexible instruments, and ergonomic tools that can potentially reduce the surgeon’s workload and facilitate precise surgery [127].
A patient-reported cosmetic outcome study involving RNSM and silicone implant reconstruction demonstrated favorable postoperative cosmetic outcomes [128]. This study highlighted how smaller incisions improve the cosmetic outcomes of breast reconstruction compared with conventional mastectomy.
In recent years, there have been an increasing number of reports on the application of robot-assisted surgery for autologous tissue reconstruction, such as LD flaps [63] and DIEP flaps [64]. Robotic LD (RLD) flap procedures can harvest muscles with smaller scars, resulting in an improved cosmetic appearance. Clemens et al [129] reported postoperative complication rates of 37.5% for LD flap compared with 16.7% for RLD flap. These include seroma (8.9% vs. 8.3%), infection (14.1% vs. 8.3%), delayed wound healing (7.8% vs. 0%), and capsular contracture (4.7% vs. 0%).
Further, the robotic DIEP (RDIEP) flap requires a smaller incision in the anterior rectus sheath compared to the traditional method (4.1 cm vs. 11.7 cm), which may reduce the risk of postoperative hernia or bulging [130]. Lee et al compared RDIEP flap with conventional DIEP flap and reported a substantial reduction in postoperative pain and hospital stay [131].
However, evidence on the safety and effectiveness of robot-assisted surgery in breast reconstruction is limited, and it has not been approved by the FDA. Cost must also be considered [132]. A clinical trial [133] is currently underway combining RNSM with RDIEP flap reconstruction. This study aims to evaluate several key parameters, including the length of the incision on the anterior rectus sheath, the time required to dissect the pedicles using robotic arms, and the weight of the harvested flap. The outcomes of this trial may provide important insights into the efficiency and safety of robotic-assisted breast reconstruction techniques. Demonstrating the safety, cosmetic outcomes, and advantages of robot-assisted surgery is key to enhancing its clinical use in breast reconstruction.
FG enrichment technique
Although FG is a minimally invasive approach for breast reconstruction, the grafted fat retention rate is inconsistent, and multiple treatments are often required. Therefore, there has been growing interest in the utilization of platelet-rich plasma (PRP), stromal vascular fraction (SVF), and adipose-derived stromal cells (ADSCs) to stimulate early angiogenesis and enhance graft retention rates.
PRP involves a small volume of plasma from which human platelets are concentrated by centrifuging whole blood samples [134]. Cervelli et al [135] used FGs for facial contouring and observed 70% fat retention with PRP compared to only 31% in the fat-only group. However, in a retrospective analysis of 42 patients who underwent FG for breast reconstruction, Salgarello et al [136] found no discernible benefit in the fat plus PRP group or any significant differences in clinical evaluation, incidence of fat necrosis, or patient preference for additional surgery.
SVF is an enzymatically treated adipose cell-depleted fraction of subcutaneous adipose tissue comprising peripheral blood-derived cells, such as macrophages and neutrophils, vascular endothelial cells, and adipose-derived stem cells. Gentile et al [137] conducted a nonrandomized trial with patients undergoing FG for breast reconstruction, including 10 patients in the fat-only control group, 13 in the PRP group, and 10 in the SVF group. Upon measuring the 1-year retention rates using MRI, the SVF (63%) and fat-only (39%) groups showed significantly improved retention rates, whereas the SVF (63%) and PRP (69%) groups revealed no significant differences.
ADSCs are mesenchymal stem cells extracted from white adipose tissue, capable of differentiating into various cell types including adipocytes and secreting numerous growth factors [138]. Kolle et al [139] conducted a randomized clinical trial involving 13 patients who underwent FG for breast augmentation. MRI evaluation after 4 months revealed significant improvement, with a retention rate of 45% in the fat-only group and 80% in the ADSC-enriched fat group.
Overall, the oncological safety of incorporating stem cells or progenitor cells into autologous FGs for reconstructive surgery after mastectomy remains unclear. Therefore, if further research can validate their safety and efficacy, this approach may represent the most minimally invasive and groundbreaking technique for breast reconstruction.
Oncoplastic surgery
Oncoplastic breast surgery, a concept proposed by Audretsch et al [140], integrates both oncological and reconstructive surgeries into breast cancer surgery. In recent years, programs have been initiated in certain countries to train surgeons to seamlessly perform breast cancer resection, partial reconstruction, and total reconstruction. In breast cancer treatment, mastectomy with reconstruction requires collaboration between surgeons; a breast surgeon performs the mastectomy, while a plastic surgeon supervises the reconstruction. This two-surgeon approach is standard in many countries, providing the advantage that specialized surgeons can handle the respective aspects of each surgery. However, if, for any reason, the breast surgeon does not refer the patient to a plastic surgeon - perhaps due to the unavailability of a plastic surgeon at the facility - the patient misses the opportunity for immediate reconstruction. Moreover, reduced reconstruction rates can also be attributed to the complexity of the increased visits to two surgeons, even if the patient was referred to a plastic surgeon [141]. These factors may contribute to persistently low global breast reconstruction rates, typically ranging from 10% to 25% [142].
In the UK, an interspecialty residency program has been in place since 2002, enabling surgeons to undergo 12 months of oncoplastic breast surgery training in specialized hospitals. This initiative has led to a significant increase in uptake rates, doubling immediate reconstructions within 3 years [143]. Similar programs have been adopted in Canada, Australia, and Brazil, where oncoplastic breast surgery is structured so that mastectomy and reconstruction are performed in a single step by a single surgeon, signifying a paradigm shift in these countries [144-146]. Such training programs not only foster collaboration between surgical specialties, but also enhance accessibility to comprehensive breast reconstruction options globally. By training breast surgeons to have integrated skills in oncoplastic surgery, these initiatives aim to increase the uptake of immediate breast reconstructions, ultimately improving patient outcomes and satisfaction.
| Conclusions | ▴Top |
This review provides an overview of the various breast reconstruction techniques and highlights the benefits and challenges associated with each technique. Both autologous tissue- and implant-based reconstructions require careful selection tailored to the specific needs of the patient, necessitating individualized treatment plans. In particular, autologous tissue reconstruction has shown the potential for higher long-term patient satisfaction and is expected to be more widely adopted in the future. Moreover, minimally invasive treatments, such as fat grafting and robotic surgery, hold the potential to further enhance patient satisfaction.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
Ryohei Katsuragi: conceptualization, investigation, resources, visualization, writing - original draft, and writing- review and editing. Cemile Nurdan Ozturk: writing - review and editing. Kohei Chida: resources, visualization, writing - review and editing. Gabriella Kim Mann: visualization and writing - review and editing. Arya Mariam Roy: writing - review and editing. Kenichi Hakamada: writing - review and editing. Kazuaki Takabe: conceptualization, investigation, visualization, writing - review and editing, and supervision. Toshihiko Satake: writing - review and editing, and supervision. All authors agreed to the final version and consented to publication.
Data Availability
The authors declare that the data supporting the findings of this review are available within the article and in the public domains, including PubMed.
Abbreviations
WHO: World Health Organization; PROs: patient-reported outcomes; FDA: Food and Drug Administration; BIA-ALCL: breast implant-associated anaplastic large cell lymphoma; ADM: acellular dermal matrices; TE: tissue expander; ASPS: American Society of Plastic Surgeons; NSM: nipple-sparing mastectomy; RT: radiation therapy; SSI: surgical site infection; BIA-SCC: breast implant-associated squamous cell carcinoma; MRI: magnetic resonance imaging; BII: breast implant illness; LDMC flap: latissimus dorsi myocutaneous flap; TRAM flap: transverse rectus abdominis myocutaneous flap; DIEP flap: deep inferior epigastric artery perforator flap; DIEA/V: deep inferior epigastric artery and vein; TUG flap: transverse upper gracilis flap; DUG flap: diagonal upper gracilis flap; SGAP flap: superior gluteal artery perforator flap; IGAP flap: inferior gluteal artery perforator flap; SIEA flap: superficial inferior epigastric artery flap; SIEA/V: superficial inferior epigastric artery and vein; LAP flap: lumber artery perforator flap; PAP flap: profunda artery perforator flap; LD flap: latissimus dorsi flap; ciNPT: closed incision negative pressure therapy; FG: fat grafting; RNSM: robotic nipple-sparing mastectomy; RLD flap: robotic LD flap; RDIEP: robotic DIEP flap; PRP: platelet-rich plasma; SVF: stromal vascular fraction; ADSCs: adipose-derived stromal cells
| References | ▴Top |
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263.
doi pubmed - Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345-353.
doi pubmed - van Bommel ACM, de Ligt KM, Schreuder K, Maduro JH, Van Dalen T, Peeters M, Mureau MAM, et al. The added value of immediate breast reconstruction to health-related quality of life of breast cancer patients. Eur J Surg Oncol. 2020;46(10 Pt A):1848-1853.
doi pubmed - Klifto KM, Tecce MG, Serletti JM, Kovach SJ. Comparison of nine methods of immediate breast reconstruction after resection of localized breast cancer: A cost-effectiveness Markov decision analysis of prospective studies. Microsurgery. 2022;42(5):401-427.
doi pubmed - Siegel EL, Whiting J, Kim Y, Sun W, Laronga C, Lee MC. Effect of surgical complications on outcomes in breast cancer patients treated with mastectomy and immediate reconstruction. Breast Cancer Res Treat. 2021;188(3):641-648.
doi pubmed - Maxwell GP, Van Natta BW, Bengtson BP, Murphy DK. Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J. 2015;35(2):145-155.
doi pubmed pmc - Federica G, Tommaso F, Alessia C, Agostino C, Florian B, Antonio G, Domenico Nicola M, et al. Use of Antimicrobial Irrigation and Incidence of Capsular Contracture in Breast Augmentation and Immediate Implant-Based Breast Reconstruction. Aesthetic Plast Surg. 2023;47(6):2345-2350.
doi pubmed pmc - Cohen JB, Carroll C, Tenenbaum MM, Myckatyn TM. Breast implant-associated infections: the role of the national surgical quality improvement program and the local microbiome. Plast Reconstr Surg. 2015;136(5):921-929.
doi pubmed - U.S. Food and Drug Administration. FDA strengthens safety requirements and updates study results for breast implants. Available at: https://www.fda.gov/news-events/press-announcements/fda-strengthens-safety-requirements-and-updates-study-results-breast-implants. [Accessed: July 16, 2024].
- Toyserkani NM, Jorgensen MG, Tabatabaeifar S, Damsgaard T, Sorensen JA. Autologous versus implant-based breast reconstruction: A systematic review and meta-analysis of Breast-Q patient-reported outcomes. J Plast Reconstr Aesthet Surg. 2020;73(2):278-285.
doi pubmed - Broyles JM, Balk EM, Adam GP, Cao W, Bhuma MR, Mehta S, Dominici LS, et al. Implant-based versus autologous reconstruction after mastectomy for breast cancer: a systematic review and meta-analysis. Plast Reconstr Surg Glob Open. 2022;10(3):e4180.
doi pubmed pmc - Stefura T, Rusinek J, Wator J, Zagorski A, Zajac M, Libondi G, Wysocki WM, et al. Implant vs. autologous tissue-based breast reconstruction: A systematic review and meta-analysis of the studies comparing surgical approaches in 55,455 patients. J Plast Reconstr Aesthet Surg. 2023;77:346-358.
doi pubmed - Kamali P, Paul MA, Ibrahim AMS, Koolen PGL, Wu W, Schermerhorn ML, Lee BT, et al. National and regional differences in 32,248 postmastectomy autologous breast reconstruction using the updated national inpatient survey. Ann Plast Surg. 2017;78(6):717-722.
doi pubmed - Kaoutzanis C, Winocour J, Unger J, Gabriel A, Maxwell GP. The evolution of breast implants. Semin Plast Surg. 2019;33(4):217-223.
doi pubmed pmc - Cronin TD, Brauer RO. Augmentation mammaplasty. Surg Clin North Am. 1971;51(2):441-452.
doi pubmed - Cocke WM, Jr. A critical review of augmentation mammoplasty with saline-filled prostheses. Ann Plast Surg. 1994;32(3):266-269.
doi pubmed - Santanelli di Pompeo F, Paolini G, Firmani G, Sorotos M. History of breast implants: Back to the future. JPRAS Open. 2022;32:166-177.
doi pubmed pmc - Berkel H, Birdsell DC, Jenkins H. Breast augmentation: a risk factor for breast cancer? N Engl J Med. 1992;326(25):1649-1653.
doi pubmed - Spear SL, Jespersen MR. Breast implants: saline or silicone? Aesthet Surg J. 2010;30(4):557-570.
doi pubmed - Heidekrueger PI, Sinno S, Hidalgo DA, Colombo M, Broer PN. Current trends in breast augmentation: an international analysis. Aesthet Surg J. 2018;38(2):133-148.
doi pubmed - Spear SL, Murphy DK, Allergan Silicone Breast Implant USCCSG. Natrelle round silicone breast implants: Core Study results at 10 years. Plast Reconstr Surg. 2014;133(6):1354-1361.
doi pubmed pmc - Gabriel A, Maxwell GP. The evolution of breast implants. Clin Plast Surg. 2015;42(4):399-404.
doi pubmed - Mohebali K, Wixtrom RN. Breast implant engineering and performance. Plast Reconstr Surg. 2018;142(4S The Science of Breast Implants):6S-11S.
doi pubmed - Loch-Wilkinson A, Beath KJ, Knight RJW, Wessels WLF, Magnusson M, Papadopoulos T, Connell T, et al. Breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: high-surface-area textured implants are associated with increased risk. Plast Reconstr Surg. 2017;140(4):645-654.
doi pubmed - Clemens MW, Miranda RN. Coming of age: breast implant-associated anaplastic large cell lymphoma after 18 years of investigation. Clin Plast Surg. 2015;42(4):605-613.
doi pubmed - Abbate O, Rosado N, Sobti N, Vieira BL, Liao EC. Meta-analysis of prepectoral implant-based breast reconstruction: guide to patient selection and current outcomes. Breast Cancer Res Treat. 2020;182(3):543-554.
doi pubmed - Holland MC, Lentz R, Sbitany H. Surgical correction of breast animation deformity with implant pocket conversion to a prepectoral plane. Plast Reconstr Surg. 2020;145(3):632-642.
doi pubmed - Caputo GG, Zingaretti N, Kiprianidis I, Zanfisi C, Domenici L, Parodi PC, Governa M. Quality of life and early functional evaluation in direct-to-implant breast reconstruction after mastectomy: a comparative study between prepectoral versus dual-plane reconstruction. Clin Breast Cancer. 2021;21(4):344-351.
doi pubmed - Scarabosio A, Contessi Negrini F, Pisano G, Beorchia Y, Castriotta L, De Francesco F, Riccio M, et al. Prepectoral direct-to-implant one-stage reconstruction with ADMs: safety and outcome in "Thin Patients". Clin Breast Cancer. 2023;23(8):e507-e514.
doi pubmed - Saldanha IJ, Cao W, Broyles JM, Adam GP, Bhuma MR, Mehta S, Dominici LS, et al. Breast reconstruction after mastectomy: a systematic review and meta-analysis. Rockville (MD): Agency for Healthcare Research and Quality (US). 2021.
doi pubmed - Plotsker EL, Stern CS, Graziano FD, Rubenstein RN, Vingan PS, Haglich K, Monge J, et al. Surgical management of textured breast implants: assessing risk and analyzing patient-reported outcomes. Plast Reconstr Surg. 2024;154(1):39-52.
doi pubmed pmc - Megevand V, Scampa M, McEvoy H, Kalbermatten DF, Oranges CM. Comparison of outcomes following prepectoral and subpectoral implants for breast reconstruction: systematic review and meta-analysis. Cancers (Basel). 2022;14(17):4223.
doi pubmed pmc - Radovan C. Tissue expansion in soft-tissue reconstruction. Plast Reconstr Surg. 1984;74(4):482-492.
doi pubmed - Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55(3):232-239.
doi pubmed - Han WY, Han SJ, Eom JS, Kim EK, Han HH. A comparative study of wraparound versus anterior coverage placement of acellular dermal matrix in prepectoral breast reconstruction. Plast Reconstr Surg. 2023;152(4):716-724.
doi pubmed - Li L, Su Y, Xiu B, Huang X, Chi W, Hou J, Zhang Y, et al. Comparison of prepectoral and subpectoral breast reconstruction after mastectomies: A systematic review and meta analysis. Eur J Surg Oncol. 2019;45(9):1542-1550.
doi pubmed - Montorfano L, Hung YC, Chaker S, Saad M, Kalmar CL, Ferri F, Higdon KK, et al. Examination of outcome disparities in reports of prepectoral and subpectoral direct-to-implant reconstruction: a systematic review and meta-analysis. Ann Plast Surg. 2023;90(5):506-515.
doi pubmed - Asaad M, Selber JC, Adelman DM, Baumann DP, Hassid VJ, Crosby MA, Liu J, et al. Allograft vs xenograft bioprosthetic mesh in tissue expander breast reconstruction: a blinded prospective randomized controlled trial. Aesthet Surg J. 2021;41(12):NP1931-NP1939.
doi pubmed - Daar DA, Abdou SA, Rosario L, Rifkin WJ, Santos PJ, Wirth GA, Lane KT. Is there a preferred incision location for nipple-sparing mastectomy? A systematic review and meta-analysis. Plast Reconstr Surg. 2019;143(5):906e-919e.
doi pubmed - Jimenez RB, Packowski K, Horick N, Rosado N, Chinta S, Koh DJ, Sobti N, et al. The timing of acute and late complications following mastectomy and implant-based reconstruction. Ann Surg. 2023;278(1):e203-e208.
doi pubmed - Galdiero M, Larocca F, Iovene MR, Francesca M, Pieretti G, D'Oriano V, Franci G, et al. Microbial evaluation in capsular contracture of breast implants. Plast Reconstr Surg. 2018;141(1):23-30.
doi pubmed - Wilson RL, Kirwan CC, Johnson RK, O'Donoghue JM, Linforth RA, Harvey JR. Breast reconstruction outcomes with and without strattice: long-term outcomes of a multicenter study comparing strattice immediate implant breast reconstruction with submuscular implant reconstruction. Plast Reconstr Surg. 2023;152(1):11-19.
doi pubmed pmc - Azouz V, Mirhaidari S, Wagner DS. Defining infection in breast reconstruction: a literature review. Ann Plast Surg. 2018;80(5):587-591.
doi pubmed - Sisco M, Kuchta K, Alva D, Seth AK. Oral antibiotics do not prevent infection or implant loss after immediate prosthetic breast reconstruction. Plast Reconstr Surg. 2023;151(5):730e-738e.
doi pubmed - Palubicka A, Jaworski R, Wekwejt M, Swieczko-Zurek B, Pikula M, Jaskiewicz J, Zielinski J. Surgical site infection after breast surgery: a retrospective analysis of 5-year postoperative data from a single center in Poland. Medicina (Kaunas). 2019;55(9):512.
doi pubmed pmc - Olsen MA, Nickel KB, Fox IK, Margenthaler JA, Wallace AE, Fraser VJ. Comparison of Wound Complications After Immediate, Delayed, and Secondary Breast Reconstruction Procedures. JAMA Surg. 2017;152(9):e172338.
doi pubmed pmc - Keech JA, Jr., Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100(2):554-555.
doi pubmed - American Society of Plastic Surgeons. BIA-ALCL Resources. By the numbers, and what they mean. Available at: https://www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-physician-resources/by-the-numbers. [Accessed: July 16, 2024].
- American Society of Plastic Surgeons. FDA provides update on Breast Implant Associated-Squamous Cell Carcinoma (BIA-SCC). Available at: https://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/fda-provides-update-on-breast-implant-associated-squamous-cell-carcinoma [Accessed: July 16, 2024].
- Tang SYQ, Israel JS, Afifi AM. Breast Implant Illness: Symptoms, Patient Concerns, and the Power of Social Media. Plast Reconstr Surg. 2017;140(5):765e-766e.
doi pubmed - Verneuil AA. Memoires de Chirurgie. Paris: G. Masson. 1887.
- Tansini I. Sopra il mio nuovo processo di amputazione della mammella. Gazz Med Ital. 1906;57(57):141.
- Robbins TH. Rectus abdominis myocutaneous flap for breast reconstruction. Aust N Z J Surg. 1979;49(5):527-530.
doi pubmed - Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42(6):645-648.
doi pubmed - Allen RJ, Tucker C, Jr. Superior gluteal artery perforator free flap for breast reconstruction. Plast Reconstr Surg. 1995;95(7):1207-1212.
doi pubmed - Allen RJ, Haddock NT, Ahn CY, Sadeghi A. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg. 2012;129(1):16e-23e.
doi pubmed - Akita S, Tokumoto H, Yamaji Y, Kosaka K, Arai M, Ando N, Maei H, et al. Scarless donor site for breast reconstruction by endoscopically assisted extended latissimus dorsi flap plus lipofilling. Plast Reconstr Surg. 2024;153(6):1209-1219.
doi pubmed pmc - de Weerd L, Elvenes OP, Strandenes E, Weum S. Autologous breast reconstruction with a free lumbar artery perforator flap. Br J Plast Surg. 2003;56(2):180-183.
doi pubmed - Beugels J, Bijkerk E, Lataster A, Heuts EM, van der Hulst R, Tuinder SMH. Nerve coaptation improves the sensory recovery of the breast in DIEP flap breast reconstruction. Plast Reconstr Surg. 2021;148(2):273-284.
doi pubmed - Arnez ZM, Khan U, Pogorelec D, Planinsek F. Breast reconstruction using the free superficial inferior epigastric artery (SIEA) flap. Br J Plast Surg. 1999;52(4):276-279.
doi pubmed - Yousif NJ. The transverse gracilis musculocutaneous flap. Ann Plast Surg. 1993;31(4):382.
doi pubmed - Allen R, Guarda H, Wall F, Dupin C, Glass C. Free flap breast reconstruction: the LSU experience (1984-1996). J La State Med Soc. 1997;149(10):388-392.
pubmed - Selber JC, Baumann DP, Holsinger CF. Robotic harvest of the latissimus dorsi muscle: laboratory and clinical experience. J Reconstr Microsurg. 2012;28(7):457-464.
doi pubmed - Gundlapalli VS, Ogunleye AA, Scott K, Wenzinger E, Ulm JP, Tavana L, Pullatt RC, et al. Robotic-assisted deep inferior epigastric artery perforator flap abdominal harvest for breast reconstruction: A case report. Microsurgery. 2018;38(6):702-705.
doi pubmed - Maxwell GP. Iginio Tansini and the origin of the latissimus dorsi musculocutaneous flap. Plast Reconstr Surg. 1980;65(5):686-692.
doi pubmed - Zheng S, Hao S, Chen J, Zhang Y, Yang B, Huang X, Liu G, et al. Latissimus dorsi flap - the main force in breast reconstruction for breast tumor in Chinese population. Front Oncol. 2023;13:1159073.
doi pubmed pmc - Haddock NT, Teotia SS. Lumbar artery perforator flap: initial experience with simultaneous bilateral flaps for breast reconstruction. Plast Reconstr Surg Glob Open. 2020;8(5):e2800.
doi pubmed pmc - Myers PL, Nelson JA, Allen RJ, Jr. Alternative flaps in autologous breast reconstruction. Gland Surg. 2021;10(1):444-459.
doi pubmed pmc - Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 1982;69(2):216-225.
doi pubmed - Blondeel PN. The sensate free superior gluteal artery perforator (S-GAP) flap: a valuable alternative in autologous breast reconstruction. Br J Plast Surg. 1999;52(3):185-193.
doi pubmed - Pacella SJ, Vogel JE, Locke MB, Codner MA. Aesthetic and technical refinements in latissimus dorsi implant breast reconstruction: a 15-year experience. Aesthet Surg J. 2011;31(2):190-199.
doi pubmed - Palve J, Luukkaala T, Kaariainen M. Comparison of different techniques in latissimus dorsi breast reconstruction: plain, immediately lipofilled, and implant enhanced. Ann Plast Surg. 2022;88(1):20-24.
doi pubmed - Murota Y, Satake T, Tsunoda Y, Muto M, Koike T, Onoda S, Maegawa J. Stacked deep inferior epigastric perforator with sequential lumbar artery perforator flaps for bilateral breast reconstruction: A case report. Microsurgery. 2022;42(8):829-834.
doi pubmed - Rozen WM, Rajkomar AK, Anavekar NS, Ashton MW. Post-mastectomy breast reconstruction: a history in evolution. Clin Breast Cancer. 2009;9(3):145-154.
doi pubmed - Grotting JC, Urist MM, Maddox WA, Vasconez LO. Conventional TRAM flap versus free microsurgical TRAM flap for immediate breast reconstruction. Plast Reconstr Surg. 1989;83(5):828-841; discussion 842-824.
doi pubmed - Seth AK, Allen RJ, Jr. Modern techniques and alternative flaps in microsurgical breast reconstruction. J Surg Oncol. 2018;118(5):768-779.
doi pubmed - Healy C, Allen RJ, Sr. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J Reconstr Microsurg. 2014;30(2):121-125.
doi pubmed - Kikuchi N, Murakami G, Kashiwa H, Homma K, Sato TJ, Ogino T. Morphometrical study of the arterial perforators of the deep inferior epigastric perforator flap. Surg Radiol Anat. 2001;23(6):375-381.
doi pubmed - Chevray PM. Breast reconstruction with superficial inferior epigastric artery flaps: a prospective comparison with TRAM and DIEP flaps. Plast Reconstr Surg. 2004;114(5):1077-1083; discussion 1084-1075.
doi pubmed - Cohen Z, Azoury SC, Nelson JA, Haglich K, Dayan JH, Matros E, Allen RJ, Jr. The preferred design of the profunda artery perforator flap for autologous breast reconstruction: transverse or diagonal? Plast Reconstr Surg Glob Open. 2023;11(8):e5188.
doi pubmed pmc - Allen RJ, Jr., Lee ZH, Mayo JL, Levine J, Ahn C, Allen RJ, Sr. The profunda artery perforator flap experience for breast reconstruction. Plast Reconstr Surg. 2016;138(5):968-975.
doi pubmed - Dayan E, Smith ML, Sultan M, Samson W, Dayan JH. The diagonal upper gracilis (DUG) flap: a safe and improved alternative to the TUG flap. Plast Reconstr Surg. 2013;132(4S-1):33-34.
- Arnez ZM, Pogorelec D, Planinsek F, Ahcan U. Breast reconstruction by the free transverse gracilis (TUG) flap. Br J Plast Surg. 2004;57(1):20-26.
doi pubmed - Craggs B, Vanmierlo B, Zeltzer A, Buyl R, Haentjens P, Hamdi M. Donor-site morbidity following harvest of the transverse myocutaneous gracilis flap for breast reconstruction. Plast Reconstr Surg. 2014;134(5):682e-691e.
doi pubmed - Dayan JH, Allen RJ, Jr. Lower Extremity Free Flaps for Breast Reconstruction. Plast Reconstr Surg. 2017;140(5S Advances in Breast Reconstruction):77S-86S.
doi pubmed - Martineau J, Kalbermatten DF, Oranges CM. Safety and efficacy of the superior gluteal artery perforator (SGAP) flap in autologous breast reconstruction: systematic review and meta-analysis. Cancers (Basel). 2022;14(18):4420.
doi pubmed pmc - Ochoa O, Chrysopoulo M, Nastala C, Ledoux P, Pisano S. Abdominal wall stability and flap complications after deep inferior epigastric perforator flap breast reconstruction: does body mass index make a difference? Analysis of 418 patients and 639 flaps. Plast Reconstr Surg. 2012;130(1):21e-33e.
doi pubmed - Qian B, Xiong L, Li J, Sun Y, Sun J, Guo N, Wang Z. A systematic review and meta-analysis on microsurgical safety and efficacy of profunda artery perforator flap in breast reconstruction. J Oncol. 2019;2019:9506720.
doi pubmed pmc - Mirzabeigi MN, Au A, Jandali S, Natoli N, Sbitany H, Serletti JM. Trials and tribulations with the inferior gluteal artery perforator flap in autologous breast reconstruction. Plast Reconstr Surg. 2011;128(6):614e-624e.
doi pubmed - Wu SS, Raymer C, Culbert A, Schafer R, Bernard S, Djohan R, Schwarz G, et al. Predictors of complications in autologous breast reconstruction using DIEP flaps: implications for management. Plast Reconstr Surg. 2023;152(4):566e-577e.
doi pubmed - Rijkx MEP, Klein DO, Hommes JE, van Mens SP, van Kuijk SMJ, Heuts EM, van der Hulst R, et al. Evidence for the use of peri- and post-operative antibiotic prophylaxis in autologous breast reconstruction: A systematic review. J Plast Reconstr Aesthet Surg. 2023;83:404-414.
doi pubmed - Mahrhofer M, Reichert R, Siegwart LC, Russe E, Schoeller T, Wechselberger G, Weitgasser L. Risk of perioperative hormonal breast cancer therapy for microvascular flap complications in breast reconstruction. J Plast Reconstr Aesthet Surg. 2023;85:143-148.
doi pubmed - Chen K, Beeraka NM, Sinelnikov MY, Zhang J, Song D, Gu Y, Li J, et al. Patient management strategies in perioperative, intraoperative, and postoperative period in breast reconstruction with DIEP-flap: clinical recommendations. Front Surg. 2022;9:729181.
doi pubmed pmc - Khansa I, Chao AH, Taghizadeh M, Nagel T, Wang D, Tiwari P. A systematic approach to emergent breast free flap takeback: Clinical outcomes, algorithm, and review of the literature. Microsurgery. 2013;33(7):505-513.
doi pubmed - Wang J, Chapman Z, Cole E, Koide S, Mah E, Overstall S, Trotter D. Use of closed incision negative pressure therapy (ciNPT) in breast reconstruction abdominal free flap donor sites. J Clin Med. 2021;10(21):5176.
doi pubmed pmc - Munro SP, Dearden A, Joseph M, O'Donoghue JM. Reducing donor-site complications in DIEP flap breast reconstruction with closed incisional negative pressure therapy: A cost-benefit analysis. J Plast Reconstr Aesthet Surg. 2023;78:13-18.
doi pubmed - Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61-68.
doi pubmed pmc - Santosa KB, Qi J, Kim HM, Hamill JB, Wilkins EG, Pusic AL. Long-term patient-reported outcomes in postmastectomy breast reconstruction. JAMA Surg. 2018;153(10):891-899.
doi pubmed pmc - Herd D. Migration, cultural transformation and the rise of black liver cirrhosis mortality. Br J Addict. 1985;80(4):397-410.
doi pubmed - Johnson L, White P, Jeevan R, Browne J, Gulliver-Clarke C, O'Donoghue J, Mohiuddin S, et al. Long-term patient-reported outcomes of immediate breast reconstruction after mastectomy for breast cancer: population-based cohort study. Br J Surg. 2023;110(12):1815-1823.
doi pubmed pmc - Lee MK, Hwang JW, Park JW, Woo KJ. Serial comparison of patient-reported outcomes of immediate breast reconstruction: direct-to-implant versus deep inferior epigastric perforator flap. Aesthetic Plast Surg. 2024;48(7):1352-1361.
doi pubmed - McCarthy CM, Klassen AF, Cano SJ, Scott A, Vanlaeken N, Lennox PA, Alderman AK, et al. Patient satisfaction with postmastectomy breast reconstruction: a comparison of saline and silicone implants. Cancer. 2010;116(24):5584-5591.
doi pubmed - Erdmann-Sager J, Wilkins EG, Pusic AL, Qi J, Hamill JB, Kim HM, Guldbrandsen GE, et al. Complications and patient-reported outcomes after abdominally based breast reconstruction: results of the mastectomy reconstruction outcomes consortium study. Plast Reconstr Surg. 2018;141(2):271-281.
doi pubmed pmc - He WY, El Eter L, Yesantharao P, Hung B, Owens H, Persing S, Sacks JM. Complications and patient-reported outcomes after TRAM and DIEP flaps: a systematic review and meta-analysis. Plast Reconstr Surg Glob Open. 2020;8(10):e3120.
doi pubmed pmc - Haddock NT, Lakatta AC, Steppe C, Teotia SS. DIEP flap versus PAP flap versus LAP flap: a propensity-matched analysis of aesthetic outcomes, complications, and satisfaction. Plast Reconstr Surg. 2024;154(4S):41S-51S.
doi pubmed - Augustin A, Morandi EM, Winkelmann S, Schoberleitner I, Egle D, Ritter M, Bauer T, et al. Long-term results after autologous breast reconstruction with DIEP versus PAP flaps based on quality of life and aesthetic outcome analysis. J Clin Med. 2023;12(3):737.
doi pubmed pmc - Opsomer D, Vyncke T, Ryx M, Stillaert F, Van Landuyt K, Blondeel P. Comparing the Lumbar and SGAP Flaps to the DIEP Flap Using the BREAST-Q. Plast Reconstr Surg. 2020;146(3):276e-282e.
doi pubmed - Neuber F. Fettransplantation. Bericht uber die Verhandlungen der Deutschen Gesellschaft fur Chirurgie. Zbl Chir. 1893;22:66.
- Heine N, Eigenberger A, Brebant V, Kempa S, Seitz S, Prantl L, Kuehlmann B. The effect of radiotherapy on fat engraftment for complete breast reconstruction using lipofilling only. Arch Gynecol Obstet. 2023;307(2):549-555.
doi pubmed pmc - Chan CW, McCulley SJ, Macmillan RD. Autologous fat transfer—a review of the literature with a focus on breast cancer surgery. J Plast Reconstr Aesthet Surg. 2008;61(12):1438-1448.
doi pubmed - Khouri RK, Rigotti G, Cardoso E, Khouri RK, Jr., Biggs TM. Megavolume autologous fat transfer: part II. Practice and techniques. Plast Reconstr Surg. 2014;133(6):1369-1377.
doi pubmed - Cogliandro A, Barone M, Tenna S, Morelli Coppola M, Persichetti P. The role of lipofilling after breast reconstruction: evaluation of outcomes and patient satisfaction with BREAST-Q. Aesthetic Plast Surg. 2017;41(6):1325-1331.
doi pubmed - Alessandri Bonetti M, Carbonaro R, Borelli F, Amendola F, Cottone G, Mazzocconi L, Mastroiacovo A, et al. Outcomes in hybrid breast reconstruction: a systematic review. Medicina (Kaunas). 2022;58(9):1232.
doi pubmed pmc - Stillaert F, Lannau B, Van Landuyt K, Blondeel PN. The prepectoral, hybrid breast reconstruction: the synergy of lipofilling and breast implants. Plast Reconstr Surg Glob Open. 2020;8(7):e2966.
doi pubmed pmc - Marchica P, Oieni S, David M, Coppola F, Rossi M, Cammarata E, Cordova A, et al. Latissimus dorsi flap and thoracodorsal artery perforator flap with immediate fat transfer (LIFT and TIFT): a retrospective study about total breast reconstruction in high-risk patients. Aesthetic Plast Surg. 2024;48(9):1745-1758.
doi pubmed - Santanelli di Pompeo F, D'Orsi G, Firmani G, Paolini G, Renzi LF, Sorotos M. Total breast reconstruction with the fat-augmented latissimus dorsi (FALD) flap: High safety in a single-center uncontrolled case series. J Plast Reconstr Aesthet Surg. 2022;75(9):3004-3013.
doi pubmed - Escandon JM, Escandon L, Ahmed A, Weiss A, Nazerali R, Ciudad P, Langstein HN, et al. Breast reconstruction using the Latissimus Dorsi Flap and Immediate Fat Transfer (LIFT): A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2022;75(11):4106-4116.
doi pubmed - Lucattelli E, Cattin F, Cipriani F, Dellachiesa L, Fogacci T, Frisoni G, Samorani D, et al. Reverse expansion following nipple sparing mastectomy: a natural, safe and effective autologous technique for breast reconstruction. Aesthetic Plast Surg. 2022;46(4):1602-1608.
doi pubmed - Homsy P, Hockerstedt A, Hukkinen K, Kauhanen S. Total breast reconstruction with lipofilling after traditional mastectomy without the use of tissue expanders. Plast Reconstr Surg. 2023;152(3):483-491.
doi pubmed pmc - Zhang X, Cai L, Yin B, Han X, Li F. Total breast reconstruction using large-volume condensed and viable fat grafting after mastectomy. J Plast Reconstr Aesthet Surg. 2021;74(5):966-973.
doi pubmed - Kavita S. Sharma JB, Adeeb Naasan, Eva M. Weiler-Mithoff Outcomes following high-versus low-volume fat transfer following breast reconstruction and conservation - the Canniesburn Experience. Eur J Plast Surg. 2020;43:269-278
- Debald M, Pech T, Kaiser C, Keyver-Paik MD, Walgenbach-Bruenagel G, Kalff JC, Kuhn W, et al. Lipofilling effects after breast cancer surgery in post-radiation patients: an analysis of results and algorithm proposal. Eur J Plast Surg. 2017;40(5):447-454.
doi pubmed pmc - Wederfoort JLM, Hebels SA, Heuts EM, van der Hulst R, Piatkowski AA. Donor site complications and satisfaction in autologous fat grafting for breast reconstruction: A systematic review. J Plast Reconstr Aesthet Surg. 2022;75(4):1316-1327.
doi pubmed - De Decker M, De Schrijver L, Thiessen F, Tondu T, Van Goethem M, Tjalma WA. Breast cancer and fat grafting: efficacy, safety and complications-a systematic review. Eur J Obstet Gynecol Reprod Biol. 2016;207:100-108.
doi pubmed - Tillo O, Nassab R, Pacifico MD. The British Association of Aesthetic Plastic Surgeons (BAAPS) gluteal fat grafting safety review and recommendations. Aesthet Surg J. 2023;43(6):675-682.
doi pubmed - Toesca A, Peradze N, Galimberti V, Manconi A, Intra M, Gentilini O, Sances D, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique. Ann Surg. 2017;266(2):e28-e30.
doi pubmed - Stefanidis D, Wang F, Korndorffer JR, Jr., Dunne JB, Scott DJ. Robotic assistance improves intracorporeal suturing performance and safety in the operating room while decreasing operator workload. Surg Endosc. 2010;24(2):377-382.
doi pubmed - Lai HW, Chen ST, Lin SL, Chen CJ, Lin YL, Pai SH, Chen DR, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: technique, preliminary results and patient-reported cosmetic outcome. Ann Surg Oncol. 2019;26(1):42-52.
doi pubmed - Clemens MW, Kronowitz S, Selber JC. Robotic-assisted latissimus dorsi harvest in delayed-immediate breast reconstruction. Semin Plast Surg. 2014;28(1):20-25.
doi pubmed pmc - Moreira A, Bailey EA, Chen B, Nelson W, Li J, Fortunato R, Nosik S, et al. A new era in perforator flap surgery for breast reconstruction: a comparative study of robotic versus standard harvest of bilateral deep inferior epigastric artery perforator flaps. J Reconstr Microsurg. 2024.
doi pubmed - Lee MJ, Won J, Song SY, Park HS, Kim JY, Shin HJ, Kwon YI, et al. Clinical outcomes following robotic versus conventional DIEP flap in breast reconstruction: A retrospective matched study. Front Oncol. 2022;12:989231.
doi pubmed pmc - Lai HW, Chen ST, Mok CW, Lin YJ, Wu HK, Lin SL, Chen DR, et al. Robotic versus conventional nipple sparing mastectomy and immediate gel implant breast reconstruction in the management of breast cancer- A case control comparison study with analysis of clinical outcome, medical cost, and patient-reported cosmetic results. J Plast Reconstr Aesthet Surg. 2020;73(8):1514-1525.
doi pubmed - ClinicalTrials.gov. Oncoplastic Entirely Robot-Assisted Approach (OPERA) - incorporating robotic surgery in both mastectomy and DIEP flap reconstruction. ClinicalTrials.gov Identifier: NCT06415526. 2024. Available at: https://clinicaltrials.gov/study/NCT06415526?cond=Breast%20Cancer&term=robotic%20DIEP&rank=1 [Accessed: September 6, 2024].
- Jun-Jiang C, Huan-Jiu X. Vascular endothelial growth factor 165-transfected adipose-derived mesenchymal stem cells promote vascularization-assisted fat transplantation. Artif Cells Nanomed Biotechnol. 2016;44(4):1141-1149.
doi pubmed - Cervelli V, Gentile P, Scioli MG, Grimaldi M, Casciani CU, Spagnoli LG, Orlandi A. Application of platelet-rich plasma in plastic surgery: clinical and in vitro evaluation. Tissue Eng Part C Methods. 2009;15(4):625-634.
doi pubmed - Salgarello M, Visconti G, Rusciani A. Breast fat grafting with platelet-rich plasma: a comparative clinical study and current state of the art. Plast Reconstr Surg. 2011;127(6):2176-2185.
doi pubmed - Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Curcio CB, Floris M, et al. A comparative translational study: the combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl Med. 2012;1(4):341-351.
doi pubmed pmc - Kapur SK, Katz AJ. Review of the adipose derived stem cell secretome. Biochimie. 2013;95(12):2222-2228.
doi pubmed - Kolle ST, Duscher D, Taudorf M, Fischer-Nielsen A, Svalgaard JD, Munthe-Fog L, Jonsson B, et al. Ex vivo-expanded autologous adipose tissue-derived stromal cells ensure enhanced fat graft retention in breast augmentation: A randomized controlled clinical trial. Stem Cells Transl Med. 2020;9(11):1277-1286.
doi pubmed pmc - Audretsch W, Rezai M, Kolotas C, Zamboglou N, Schnabel T, Bojar H. Tumor-specific immediate reconstruction in breast cancer patients. Seminars in Plastic Surgery. 1998;11:71-100.
- Shaterian A, Saba SC, Yee B, Tokin C, Mailey B, Dobke MK, Wallace AM. Single dual-trained surgeon for breast care leads to higher reconstruction rates after mastectomy. World J Surg. 2013;37(11):2600-2606.
doi pubmed - Lai HW, Lin J, Sae-Lim C, Lin YJ, Chen DR, Lai YC, Lin SL, et al. Oncoplastic and reconstructive breast surgeon performance and impact on breast reconstructions: Clinical outcomes, learning curve, and patients' satisfaction. Surg Oncol. 2023;47:101920.
doi pubmed - Cardoso MJ, Macmillan RD, Merck B, Munhoz AM, Rainsbury R. Training in oncoplastic surgery: an international consensus. The 7th Portuguese Senology Congress, Vilamoura, 2009. Breast. 2010;19(6):538-540.
doi pubmed - Peiris L, Olson D, Kelly D. Oncoplastic and reconstructive breast surgery in Canada: breaking new ground in general surgical training. Can J Surg. 2018;61(5):294-299.
doi pubmed pmc - Yunaev M, Hingston G. Oncoplastic breast surgery: a regional Australian 2012 fellowship experience. ANZ J Surg. 2013;83(9):624-629.
doi pubmed - Businaro Fernandes Joao T, de Oliveira VM, Bagnoli F, Bastos MCS, Rinaldi JF, Brenelli FP, Mateus EF. How well are Brazilian mastologists (breast surgeons) trained in breast reconstruction and oncoplastic surgery? A study of the impact of a breast reconstruction and oncoplastic surgery improvement course. Front Oncol. 2023;13:1139461.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.