| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://wjon.elmerpub.com |
Original Article
Volume 16, Number 1, February 2025, pages 95-103
Impact of 2021 World Health Organization Grading, Peritumoral Edema, and Radiotherapy on the Recurrence of a Grossly Resected Intracranial Meningiomas: A Ten-Year Follow-Up Study
Alaa Alkhotania, Saleh Baeesab , Maryam Alshanqitic, Taghreed Alsinanid, Ahmed Najjare, Shadi Alkhayyatf, Awab Tayyibg, Zayed Jastaniahh, Abdulrahman J. Sabbaghi, Nadeem S. Buttj, Hussain A. Alamoudik, Mohammed Alharbik, Basem Bahakeeml, Maher Kurdim, n
aDepartment of Pathology, College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
bDepartment of Neurosciences, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
cDepartment of Neurosurgery, King Fahad Hospital, Madinah, Saudi Arabia
dDepartment of Neurosurgery, King Fahad Hospital, Jeddah, Saudi Arabia
eDepartment of Surgery, Faculty of Medicine, Taibah University, Madina, Saudi Arabia
fDepartment of Internal Medicine, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
gDepartment of Pathology, Faculty of Medicine, University of Jeddah, Jeddah, Saudi Arabia
hDepartment of Internal Medicine, Faculty of Medicine, King Abdulaziz University, Rabigh, Saudi Arabia
iDepartment of Surgery, Faculty of Medicine. King Abdulaziz University, Jeddah, Saudi Arabia
jDepartment of Family and Community Medicine, Faculty of Medicine. King Abdulaziz University, Rabigh, Saudi Arabia
kOncology Center, First Health Cluster, East Jeddah Hospital, Jeddah, Saudi Arabia
lDepartment of Internal Medicine, College of Medicine, Umm Al-Qura University, Mecca, Saudi Arabia
mDepartment of Pathology, Faculty of Medicine, King Abdulaziz University, Rabigh, Saudi Arabia
nCorresponding Author: Maher Kurdi, Department of Pathology, Faculty of Medicine, King Abdulaziz University, Rabigh, Saudi Arabia
Manuscript submitted November 6, 2024, accepted December 30, 2024, published online January 7, 2025
Short title: WHO Grading, PTE, RT and Meningioma Recurrence
doi: https://doi.org/10.14740/wjon1999
| Abstract | ▴Top |
Background: The significance of histological grading and peritumoral edema (PTE) in predicting intracranial meningioma recurrence among Saudis is often neglected. This study aimed to evaluate the impact of these factors over a 10-year follow-up period.
Methods: A retrospective cohort of 124 patients with intracranial meningioma was analyzed over the period from 2011 to 2021. All patients underwent gross total resection (GTR) of the tumor. Post-surgical radiotherapy (RT) was offered to patients with grade II-III meningiomas. The impact of histological grading, PTE, and RT on the recurrence-free interval (RFI) was investigated.
Results: The mean age of the patients was 49 years (range: 18 - 84), with 87 females (70.2%) and 37 males (29.8%). Most tumors (88.7%, n = 110) were supratentorial, while 11.3% (n = 14) were infratentorial. The World Health Organization (WHO) grading classified 101 tumors (81.5%) as grade I, 17 (13.7%) as grade II, and six (4.8%) patients as grade III. Grading was significantly associated with RFI (P = 0.013), with grade I meningiomas having the slowest recurrence. The overall recurrence rate was 16.9%, with 38.1% (n = 8) of grade I and 61.9% (n = 13) of grade II-III meningiomas recurring within 5 years post-GTR and RT. There was no significant difference in RFI between RT-exposed and non-exposed patients (P = 0.15). PTE was present in 76 cases (61.3%) and absent in 48 (38.7%), significantly affecting RFI (P = 0.014), with shorter RFI in PTE cases. Overall, 95.2% (n = 118) of patients survived, while 4.8% (n = 6) died; five had grade II-III, and one had grade I meningioma.
Conclusions: Totally, resected intracranial meningiomas with grade II-III features and PTE were associated with earlier tumor recurrence and poorer patient survival. Post-surgical RT had an insignificant effect on the RFI.
Keywords: Meningioma; Epidemiology; Peritumoral edema; Grading; Recurrence
| Introduction | ▴Top |
Meningiomas are the most common primary tumors of the central nervous system (CNS), comprising 37.6% of all primary CNS tumors and 53.3% of benign CNS tumors [1]. These typically benign, slow-growing, extra-axial neoplasms are believed to originate from arachnoid cup cells [1, 2]. The incidence of meningiomas increases with age, with the median age at diagnosis being 66 years old [3]. The incidence rate of meningiomas is 18.69 per 100,000 in individuals aged 40 and above and 0.16 per 100,000 in those aged 0 - 19. Meningiomas are more common in females than males [3]. The 2021 World Health Organization (WHO) fifth edition classified meningiomas into 15 subtypes based on different histological gradings, which are determined by mitotic rate, brain invasion, and specific histological features [4]. Atypical meningioma (grade 2) is diagnosed by the presence of 4 - 19 mitotic figures per 10 high-power fields (HPFs) or unequivocal brain invasion. Certain histomolecular features, such as choroid type, TERT promoter mutations, or CDKN2A/B deletions, now result in higher-grade classifications [4, 5]. Rhabdoid and papillary meningiomas are no longer automatically classified as grade III; their grading is based on the same criteria used for other meningioma types [4, 6].
Histological grading is considered the most reliable prognostic factor of meningiomas, followed by the extent of tumor resection (Simpson grade) [6]. For instance, partially resected grade I meningioma may recur slower than totally resected grade II meningioma. Other factors associated with an increased risk of recurrence are intracranial invasion, bone involvement, venous sinus invasion, and peritumoral brain edema (PTE) [6-9]. These factors are not always associated with tumor progression or recurrence and may depend on tumor grading and the extent of tumor resection. PTE is common in meningioma, and its relationship with prognosis is still underestimated [10]. Frati et al found that a higher edema/tumor ratio was associated with a higher frequency of recurrence but not with the size of the contrast-enhancing lesion [11]. The combination of necrosis and brain invasion in atypical meningioma was also found to be a strong predictor of tumor recurrence, regardless of the extent of tumor resection or the effect of radiotherapy (RT) [12]. Gross total resection (GTR) is also associated with a lower risk of recurrence compared to subtotal resection (STR) [13]. This was demonstrated in a meta-analysis of 18 observational studies involving 1,589 patients with grade II meningiomas [13]. Additionally, a Ki-67 proliferative index greater than 3% was associated with a higher risk of recurrence in any type of meningioma [13].
For grade II and III meningiomas, post-surgical RT had an insignificant effect on tumor recurrence [13]. Pollock et al found no difference in the 7-year progression-free survival (PFS) rate between STR and GTR [14]. Recently, molecular markers like TERT and CDKN2A/B, which classify meningiomas into higher grades, have also become essential prognostic indicators for all meningiomas [15]. The recurrence rate of meningiomas after surgery was estimated to be between 15% and 40% [16]. The recurrence rate of WHO grade I meningioma after GTR is relatively less than partially resected grade I meningioma [16]. The 5-year recurrence rates following Simpson grade I GTR for WHO grade I, II, and III meningiomas are 7-20%, 30-50%, and 70-80%, respectively [17]. The 10-year overall survival (OS) rates for WHO grade I, II, and III tumors are 85%, 55%, and 0%, respectively [5].
In Saudi Arabia, clinical data on the incidence and prevalence of intracranial meningiomas remain limited. Most studies have focused on specific regional populations. A study by Mohammed et al examined 227 brain tumor cases in the Madinah region, revealing that about 30% were meningiomas, identifying it as the most common primary CNS tumor [18]. A larger study conducted in 2020 at the National Neurological Institute of Saudi Arabia analyzed 992 cases of primary CNS tumors over 10 years [19]. Non-malignant tumors, including meningiomas, were predominant in the adult population. Kurdi et al conducted the first tumor registry for the Western Province of Saudi Arabia, describing primary CNS tumors’ distribution and epidemiological patterns [20]. Their retrospective analysis included 663 patients. Meningiomas were identified as the second most common primary brain tumor, accounting for 15%, with an overall recurrence rate of 8% [20]. Another 2024 retrospective analysis of 74 Saudi patients with grade II meningiomas in the central region found no significant difference in recurrence rates across different types of grade II meningiomas [21].
Current studies on meningiomas face several limitations, including limited data on molecular markers, comorbidities, post-surgical treatments, and inadequate long-term follow-up to assess recurrence and survival. Regional differences in healthcare systems and treatment protocols, particularly in Saudi Arabia, further hinder generalization.
Our study aims to evaluate the impact of 2021 WHO grading, PTE, and post-surgical RT on the recurrence and intracranial meningiomas in Saudi patients of Arabic descent.
| Materials and Methods | ▴Top |
Patients’ stratification
Patients were randomly selected from two tertiary hospitals in the city of Jeddah in Saudi Arabia during the period between 2011 and 2021. The study was conducted according to the guidelines of the Declaration of Helsinki on human subjects and approved by the Biomedical Ethics Committees at King Absulaziz University under general ethical agreement between King Faisal Specialist Hospital and Research Center, Jeddah (CA number: 2020-6) and King Abdulaziz University Jeddah (Institutional Review Board (IRB) number: 190-19). The 124 eligible patients enrolled in this study were adults of both genders with histologically proven intracranial meningioma. The clinical data were retrieved from the hospital records, including radiological features seen through magnetic resonance imaging (MRI), histopathological grading, post-surgical treatment, time to recurrence, and patient survival.
Study design with inclusion and exclusion criteria
Patients with intracranial meningioma in our study were classified into grade I-III meningiomas according to the fifth edition of the WHO classification of CNS tumors [4]. Sex and tumor location were key determinants in our research. Supratentorial locations included meningiomas located on the cerebrum and attached to the dura. In contrast, infratentorial locations encompassed meningiomas in the posterior fossa, cerebellum, or cerebellopontine angle (CPA), also attached to the dura. Grade I meningioma was diagnosed when there was no brain invasion, and mitotic activity was less than four per 10 HPFs. Grade II meningioma was characterized by the presence of unequivocal brain invasion, elevated mitotic activity (four to 19 per 10 HPFs), or specific histological features or subtypes, such as chordoid meningioma. Grade III meningioma was identified when mitotic activity exceeded 19 per 10 HPFs or when the tumor exhibited distinct histological characteristics, such as frank anaplasia or extensive rhabdoid features [5]. All patients in our study underwent GTR, and the post-surgical RT were only offered to 19 patients with grade II-III meningiomas. None of the patients have received chemotherapy. Tumor recurrence was estimated by identifying newly developed lesions after contrast-enhanced brain MRI. Recurrence-free interval (RFI) was estimated from the first day after surgery, including the period of post-surgical RT, to the first day of tumor recurrence. Our study has neglected patient comorbidities and the extent of radiation dose used.
Statistical interpretation
Data were described as frequencies and percentages. Decision tree analysis with Chi-square tests was applied to visually represent the complex interactions among sex, tumor location, WHO grading, and post-surgical RT in relation to RFI. Survival analyses were performed using the Kaplan-Meier (KM) method with two-sided log-ranks to compare the RFI across different WHO grades and the presence of PTE. Statistical significance was determined at the P value < 0.05 level. All statstics were performed using IBM SPSS1 version 24 statistical software (IBM Corp., Armonk, NY, USA).
| Results | ▴Top |
The decision tree illustrated in the Figure 1 presents data on meningioma prevalence and recurrence among 124 individuals, categorized by sex, tumor location, WHO grading, and post-surgical treatment. The mean age of the patients was 49 years (standard deviation (SD) 13.2; range: 18 - 84 years), segregated into 87 (70.2%) females and 37 (29.8%) males (Table 1). Tumor locations are classified as infratentorial and supratentorial. WHO grades ranged from I to III, and post-surgical treatments included “none” or “radiation”. About 88.7% (n = 110) of the tumors were supratentorial (78 females and 32 males), and 11.3% (n = 14) were infratentorial (nine females, five males). Supratentorial region was the most predominant location for meningiomas in both genders (Fig. 1). The most common supratentorial location was frontal lobe (n = 40, 32.2%), followed by sphenoidal wing (n = 12, 9.7%) (Table 1). WHO grading revealed that 101(81.5%) tumors were classified as grade I (79 females and 22 males), 17 (13.7%) tumors as grade II (eight females and nine males), and six (4.8%) tumors as grade III only males (Table 1, Fig. 1). Histologically, the most frequent grade I meningiomas was meningothelial subtype (n = 93, 75%), followed by atypical grade II meningiomas (n = 16, 12.9%). All rhabdoid meningiomas in our study (n = 4, 3.2%) showed grade III features. PTE was observed in 76 (61.3%) cases, while 48 (38.7%) cases had no PTE (Table 1).
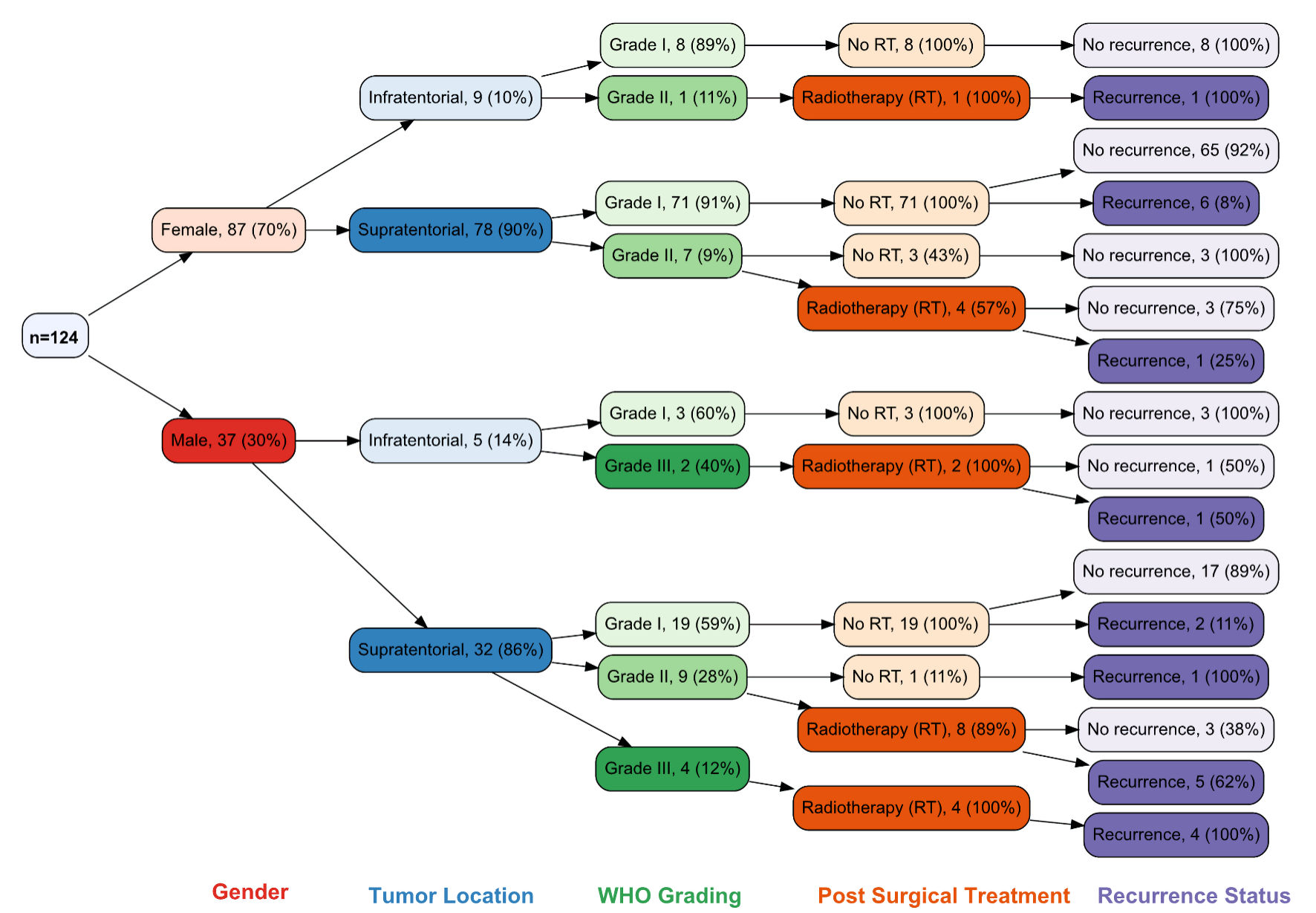 Click for large image | Figure 1. Decision tree of the 124 patients enrolled in this study was segregated by sex, tumor location, WHO grading, treatment, and recurrence-free interval (RFI). WHO: World Health Organization; RT: radiotherapy. |
 Click to view | Table 1. Characteristics of 124 Patients With Intracranial Meningioma |
Recurrence and survival
All patients underwent GTR of the tumor. Post-surgical RT was offered to patients with grade II and grade III meningiomas (Fig. 1). There was no statistically significant difference in the mean age at diagnosis among patients with and without tumor recurrence (P value = 0.594) (Table 2). However, recurrence was observed to be higher in males with supratentorial tumors (38%, n = 13), whereas females showed a recurrence rate of 9% for the same location (Fig. 1). Different meningioma grades showed a significant association with tumor recurrence (P value = 0.013), with grade I meningiomas exhibiting the slowest recurrence compared to grade II and III meningiomas (Fig. 2, Table 2). Over the 10-year follow-up period, approximately 90.3% (n = 93) of patients with grade I meningioma showed no tumor recurrence, representing the majority of nonrecurrent cases (Table 2).
 Click to view | Table 2. The Relationship Between Patients’ Age, 2021 WHO Grading, and the Recurrence Rate Among 124 Meningioma Cases |
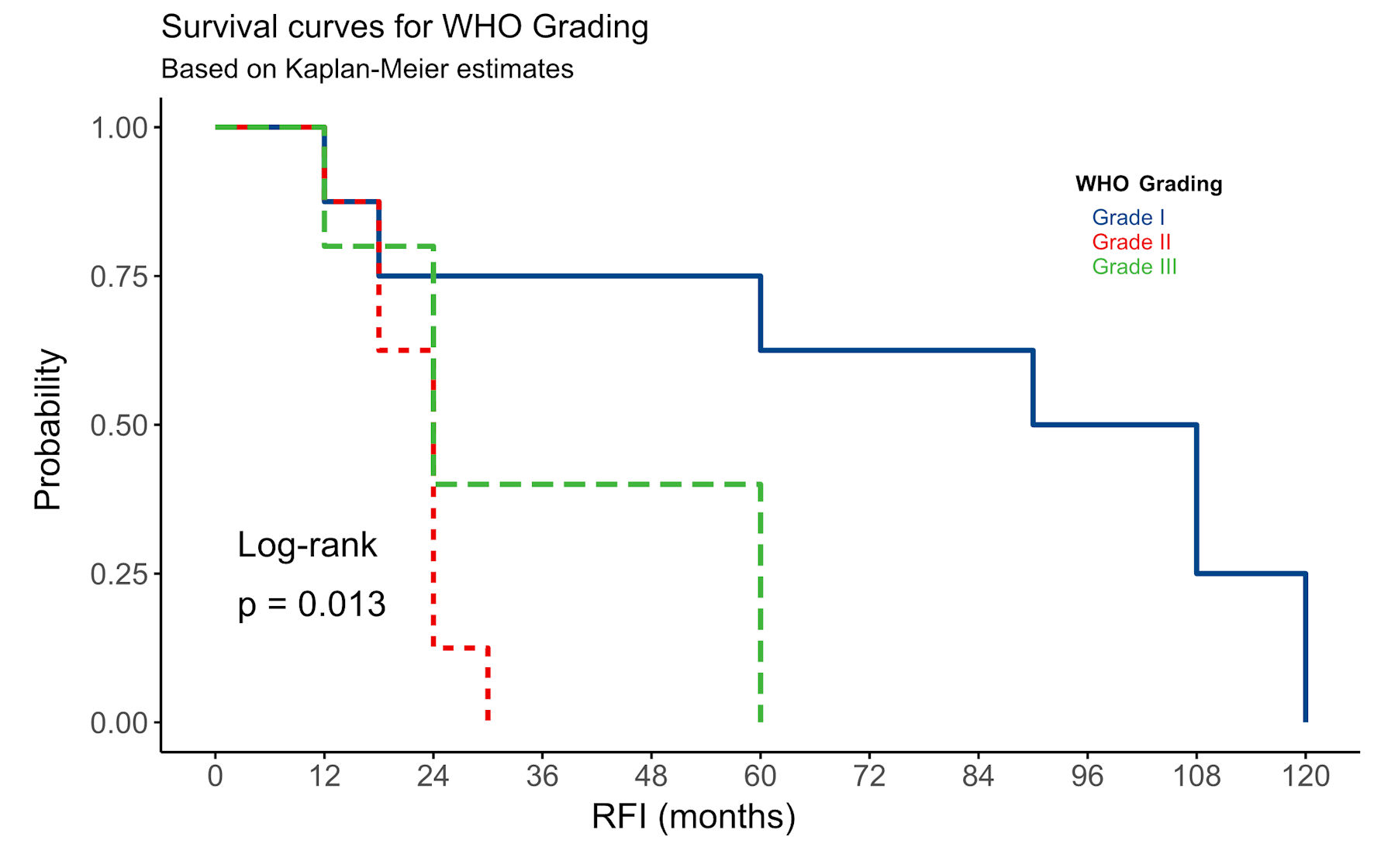 Click for large image | Figure 2. The relationship between WHO grading and RFI among all 124 patients with intracranial meningiomas. RFI: recurrence-free interval; WHO: World Health Organization. |
Our study’s overall recurrence rate among all meningioma cases was 16.9%. Among all recurrent tumors in our study, 38.1% (n = 8) of grade I meningiomas exhibited tumor recurrence during the 10-year follow-up period (Table 2). Except for two patients who experienced recurrence within 2 years of GTR with imaging scans showing extensive PTE, all other cases recurred after 5 years post-GTR (Fig. 1). For the higher-grade meningiomas, nine cases (8.7%) of grade II and one case of grade III showed no recurrence over the 10-year follow-up period, regardless of post-surgical RT (Fig. 1, Table 2). In contrast, 13 cases (61.9%) of grade II and grade III meningiomas experienced tumor recurrence within 5 years of GTR and post-surgical RT (Table 2). While there was a statistically significant difference in RFI among grade II-III meningiomas (Fig. 2), the difference in RFI between patients who received RT and those who did not was not statistically significant (P value = 0.15). However, post-surgical RT was correlated with increased recurrence in certain instances, notably in male patients with grade III tumors (Fig. 1). The presence or absence of PTE in all meningioma patients showed a significant difference in RFI (P value = 0.014) (Fig. 3). Meningiomas with PTE had a shorter RFI and earlier tumor recurrence compared to those without PTE. Overall, the survival data showed that 118 (95.2%) patients survived, while six (4.8%) patients died (Table 1). Of those who died, five patients had grade II-III meningiomas, and one had a grade I meningioma.
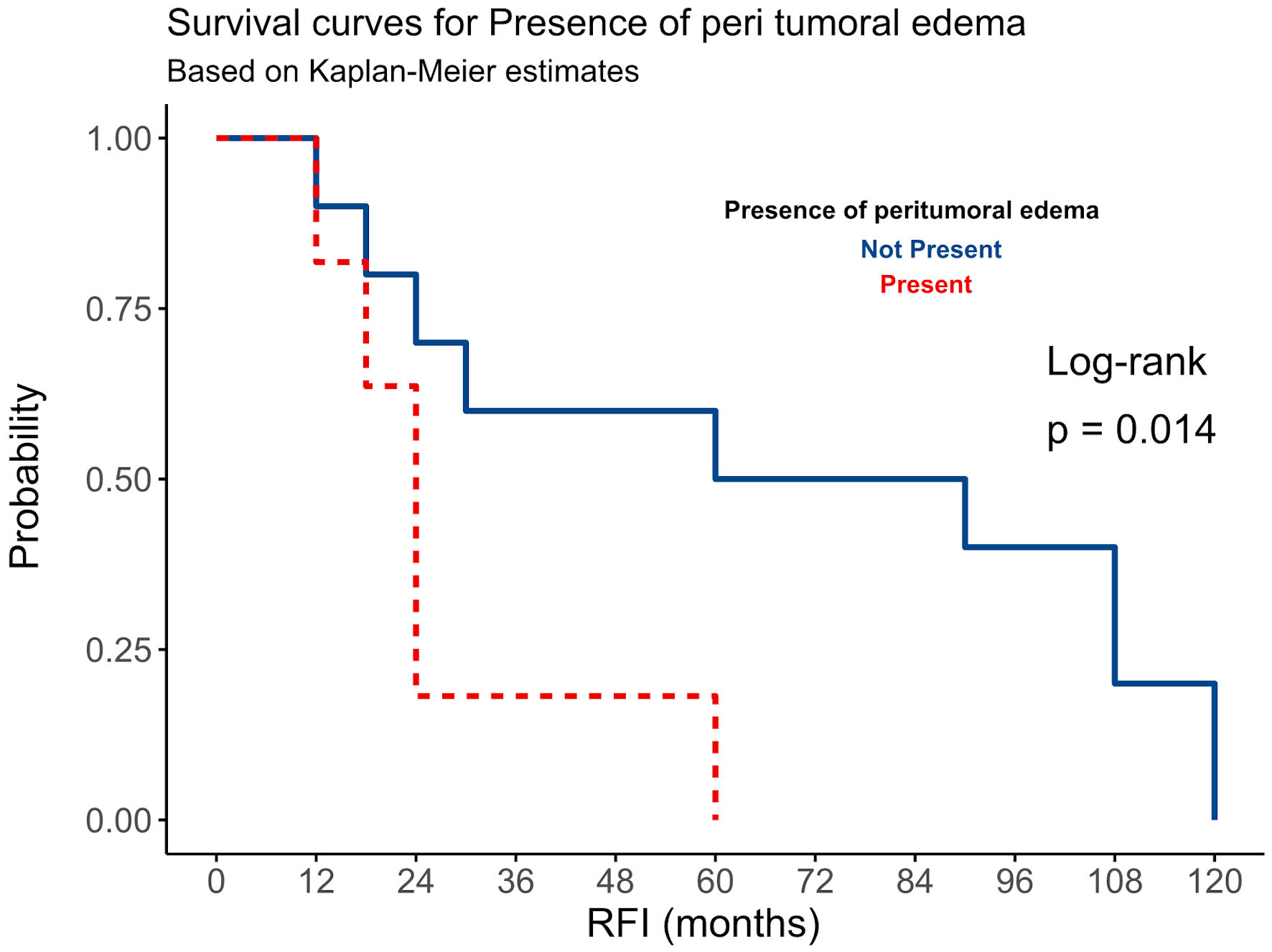 Click for large image | Figure 3. The relationship between peritumoral edema (PTE) and RFI among all 124 patients with intracranial meningiomas. RFI: recurrence-free interval. |
| Discussion | ▴Top |
Patients with intracranial meningioma may experience no recurrence for several years, especially for grade I tumors. This is primarily determined by the extent of surgical resection. However, other factors in literature were explored. In our study we found that recurrence was higher in males with supratentorial tumors. Higher recurrence rates in males with supratentorial meningiomas may stem from several factors. Biological differences linked to sex suggest that males are at greater risk for recurrence, as studies - including the Brain Tumor Registry of Japan - identify male sex as an independent risk factor for meningioma recurrence [22]. Supratentorial tumor locations, particularly on the convexity, are associated with aggressive behavior and challenges in achieving complete resection, contributing to higher recurrence rates [23]. Molecular alterations, such as increased DNA methylation and global copy number changes in recurrent meningiomas, further highlight inherent biological aggressiveness [24]. GTR is usually associated with slow tumor recurrence and longer survival compared to STR [12, 13, 17, 25, 26]. However, a subset of patients, mainly those with grade II-III meningiomas, may experience recurrence not only at the original resection site but also outside the resection cavity [27]. As a result, post-surgical RT has been used as an additional treatment for grade II and III meningiomas, with the primary goal of reducing tumor recurrence rates [27]. Despite ongoing research, the role and clinical impact of RT for meningiomas remains unclear due to conflicting findings [28]. Sanikommu et al found that post-surgical RT has an insignificant effect on the recurrence process [13]. Conversely, a recent study demonstrated that adjuvant fractionated RT is linked to improved PFS in intracranial meningiomas compared to salvage RT [29]. Although WHO grading remains the primary predictor of patient outcomes, other independent factors may influence the likelihood of tumor recurrence, including age, intra-tumoral necrosis, PTE, progesterone levels in females, clinical Simpson grading, and the Ki-67 proliferative index [7, 30]. Recently, genetic mutations in the tumor and the patient’s race have also been investigated as potential predictors of meningioma recurrence [5, 31].
Our cohort investigated the impact of WHO histological grading, PTE, and post-surgical RT on the recurrence of 124 Saudi patients with totally resected intracranial meningioma over a 10-year follow-up period. We found that WHO grading is the most significant factor for tumor recurrence and patient survival among Saudi patients (Fig. 2), consistent with other studies’ findings [9, 32]. Grade I meningiomas had a slower recurrence rate compared to grade II-III meningiomas. Among all grade I cases, approximately 92% of patients showed no recurrence over the 10-year follow-up, while 8% (8/101) experienced recurrence. Of these eight cases, all but two recurred after 5 years post-GTR. The two earlier recurrences occurred within 2 years and were associated with significant PTE on imaging. In contrast, grade II and III meningiomas recurred earlier than grade I (Fig. 2). About 56% (13/23) of grade II-III meningiomas recurred within 5 years after GTR and post-surgical RT (Fig. 1). Thus, RT did not significantly reduce tumor recurrence in grade II-III meningioma cases in our study (P value > 0.05). This finding aligns with evidence suggesting that post-surgical RT may not impact PFS in patients with higher-grade meningiomas [13], or that the tumor bed may be resistant to radiation. Grade III tumors, brain invasion, and high Ki-67 were found to be factors to predict treatment failure in these patients [33]. Variability in treatment protocols, including RT dosage and delivery methods, might also reduce its effectiveness in targeting residual aggressive tumor cells. Additionally, the inherent resistance of high-grade tumors to RT, coupled with factors like incomplete resection or extensive PTE, could overshadow the potential benefits of RT.
On the other hand, PTE showed a significant impact on tumor recurrence (RFI) in both low-grade and high-grade meningiomas, with those exhibiting PTE recurring earlier than those without PTE (Fig. 3). A study by Frati et al on 216 patients with intracranial meningioma found that PTE is associated with a high recurrence rate [11]. PTE refers to the swelling of the brain tissue surrounding the tumor (Fig. 4). This swelling may increase intracranial pressure and cause herniation if not adequately managed and controlled [34]. Although PTE in meningiomas is frequent, the mechanisms behind its development are not clearly understood [35]. The extent of edema can vary depending on the size and location of the meningioma. Larger tumors with high vascularization and close to sagittal sinus may cause more edema [36, 37]. Supratentorial meningiomas were found to have more extensive PTE compared to infratentorial meningiomas, suggesting that the latter may be diagnosed and treated earlier. The secretory subtype of meningioma is also commonly linked to PTE; however, our study did not confirm this association. It is also believed that tumor growth and local tissue hypoxia stimulate angiogenesis, contributing to the development of PTE [38, 39]. The angiogenic protein vascular endothelial growth factor (VEGF-A) plays a crucial role in promoting blood vessel growth and increasing vascular permeability [40, 41]. Meningiomas with strong VEGF immunostaining were associated with a higher incidence of PTE and a greater edema index compared to VEGF-negative meningiomas [42]. Some studies have also suggested a potential correlation between meningioma grade and the presence of mast cells, though the findings remain inconclusive [43]. Other theories focus on matrix metalloproteinases (MMPs) and the blood-brain barrier (BBB). In meningiomas, MMP-9 expression appears to be linked to the development of PTE [44]. The integrity of the BBB is compromised by meningiomas, allowing fluid to enter the surrounding brain tissue [45]. Additionally, damage to the BBB caused by smoking may contribute to increased PTE volume [11]. Patients with PTE are more likely to have preoperative symptoms, neurological deficits, and post-surgical complications. Imaging studies, such as MRI scans, are often used to assess the presence and extent of PTE in meningioma [46].
 Click for large image | Figure 4. Peritumoral edema (PTE) around the intracranial meningioma. The left image is created using an artificial intelligence (AI) program, ChatGPT 4-DALL 2 plus. |
PTE is a stronger predictor of tumor recurrence than RT due to its significant impact on the tumor microenvironment, surgical resection, and residual disease. PTE reflects increased vascular permeability and inflammatory responses, creating a pro-tumorigenic environment that facilitates tumor invasion and growth. It is often associated with larger, more invasive tumors, which are harder to completely resect, leaving residual cells that contribute to recurrence [38, 39]. While RT targets microscopic residual disease, its efficacy may be diminished by tumor resistance, particularly in aggressive tumors linked to PTE.
Limitations
The limitations in our study are acknowledged, which include its retrospective design, potential selection bias, and the lack of detailed information on key factors such as the extent of PTE, radiation dose, and patient comorbidities. Additionally, the study was constrained by a limited number of cases with higher-grade tumors.
Conclusions
The probability of recurrence of intracranial meningioma poses a significant challenge in clinical practice. Our research indicates that meningioma recurrence rates are higher in grade II-III tumors compared to grade I and in tumors that are associated with PTE. Additionally, post-surgical RT did not affect recurrence rates in high-grade meningiomas.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no relevant conflict of interest to disclose.
Informed Consent
Informed consent is not required in this study.
Author Contributions
Alaa Alkhotani and Maher Kurdi: study design, conceptualization, methodology. Saleh Baeesa: clinical data analysis and interpretation. Maryam Alshanqiti: literature review, editing and writing. Taghreed Alsinani, Ahmed Najjar, Shadi Alkhayyat, Hussain A. Alamoudi, and Abdulrahman J. Sabbagh: patients’ data and clinical analysis. Awab Tayyib: literature review. Zayed Jastaniah, Basem Bahakeem, Mohammed Alharbi: methodology. Nadeem Butt: statistical analysis. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author (MK) on reasonable request.
| References | ▴Top |
- Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020;22(12 Suppl 2):iv1-iv96.
doi pubmed - Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307-314.
doi pubmed - Ogasawara C, Philbrick BD, Adamson DC. Meningioma: a review of epidemiology, pathology, diagnosis, treatment, and future directions. Biomedicines. 2021;9(3):319.
doi pubmed - Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231-1251.
doi pubmed - Goldbrunner R, Stavrinou P, Jenkinson MD, Sahm F, Mawrin C, Weber DC, Preusser M, et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021;23(11):1821-1834.
doi pubmed - Nowak A, Dziedzic T, Krych P, Czernicki T, Kunert P, Marchel A. Benign versus atypical meningiomas: risk factors predicting recurrence. Neurol Neurochir Pol. 2015;49(1):1-10.
doi pubmed - Behling F, Hempel JM, Schittenhelm J. Brain invasion in meningioma-a prognostic potential worth exploring. Cancers (Basel). 2021;13(13):3259.
doi pubmed - Champeaux C, Houston D, Dunn L, Resche-Rigon M. Intracranial WHO grade I meningioma: a competing risk analysis of progression and disease-specific survival. Acta Neurochir (Wien). 2019;161:2541-2549.
doi pubmed - Shin HK, Park JH, Cho YH, Kim YH, Hong SH, Kim JH, Roh SW, et al. Risk factors for high-grade meningioma in brain and spine: systematic review and meta-analysis. World Neurosurg. 2021;151:e718-e730.
doi pubmed - Zhang R, Chen X, Cai J, Jiang P, Chen Y, Sun B, Song Y, et al. A novel MRI-based risk stratification algorithm for predicting postoperative recurrence of meningioma: more benefits to patients. Front Oncol. 2021;11:737520.
doi pubmed - Frati A, Armocida D, Arcidiacono UA, Pesce A, D'Andrea G, Cofano F, Garbossa D, et al. Peritumoral brain edema in relation to tumor size is a variable that influences the risk of recurrence in intracranial meningiomas. Tomography. 2022;8(4):1987-1996.
doi pubmed - Garcia-Segura ME, Erickson AW, Jairath R, Munoz DG, Das S. Necrosis and brain invasion predict radio-resistance and tumor recurrence in atypical meningioma: a retrospective cohort study. Neurosurgery. 2020;88(1):E42-E48.
doi pubmed - Sanikommu S, Panchawagh S, Eatz T, Lu VM, Rodrigues PB, Abdelsalam A, Gurses ME, et al. Recurrence of atypical and anaplastic intracranial Meningiomas: a meta-analysis of risk factors. Clin Neurol Neurosurg. 2024;244:108450.
doi pubmed - Pollock BE, Stafford SL, Utter A, Giannini C, Schreiner SA. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003;55(4):1000-1005.
doi pubmed - Wach J, Basaran AE, Arlt F, Vychopen M, Seidel C, Barrantes-Freer A, Muller W, et al. CDKN2A/B deletions are strongly associated with meningioma progression: a meta-analysis of individual patient data. Acta Neuropathol Commun. 2023;11(1):189.
doi pubmed - Zhao L, Zhao W, Hou Y, Wen C, Wang J, Wu P, Guo Z. An overview of managements in meningiomas. Front Oncol. 2020;10:1523.
doi pubmed - Haddad AF, Young JS, Kanungo I, Sudhir S, Chen JS, Raleigh DR, Magill ST, et al. WHO grade I meningioma recurrence: identifying high risk patients using histopathological features and the MIB-1 index. Front Oncol. 2020;10:1522.
doi pubmed - Mohammed AA, Hamdan AN, Homoud AS. Histopathological profile of brain tumors: a 12-year retrospective study from Madinah, Saudi Arabia. Asian J Neurosurg. 2019;14(4):1106-1111.
doi pubmed - Almutrafi A, Bashawry Y, AlShakweer W, Al-Harbi M, Altwairgi A, Al-Dandan S. The epidemiology of primary central nervous system tumors at the National Neurologic Institute in Saudi Arabia: a ten-year single-institution study. J Cancer Epidemiol. 2020;2020:1429615.
doi pubmed - Kurdi M, Butt NS, Baeesa S, Alghamdi B, Maghrabi Y, Bardeesi A, Saeedi R, et al. Epidemiological distribution of primary central nervous system tumors in the Western Province of Saudi Arabia: a local registry from neuroscience-affiliated centers. Epidemiol Health. 2021;43:e2021037.
doi pubmed - Ajlan A, Almeshari S, Basindwah S, Aljohani M, Alharbi Y, Aldhowaihy F, Alkhaldi H, et al. Atypical meningiomas compared to other WHO Grade 2 meningiomas: Histological features and prognosis. Neurosciences (Riyadh). 2024;29(2):96-102.
doi pubmed - Oya S, Ikawa F, Ichihara N, Wanibuchi M, Akiyama Y, Nakatomi H, Mikuni N, et al. Male sex and presence of preoperative symptoms are associated with early recurrence of WHO grade I meningiomas after surgical resection: analysis from the nationwide Brain Tumor Registry of Japan. Neurosurg Rev. 2022;46(1):10.
doi pubmed - Sun C, Dou Z, Wu J, Jiang B, Iranmanesh Y, Yu X, Li J, et al. The preferred locations of meningioma according to different biological characteristics based on voxel-wise analysis. Front Oncol. 2020;10:1412.
doi pubmed - Pugazenthi S, Patel B, English CW, Leidig WA, McGeehan KP, McCornack CR, Mok S, et al. Multiomic and clinical analysis of multiply recurrent meningiomas reveals risk factors, underlying biology, and insights into evolution. Sci Adv. 2024;10(43):eadn4419.
doi pubmed - Pessina F, Navarria P, Clerici E, Soffietti R, Nibali MC, Ruda R, Riva M, et al. Intracranial meningiomas: a systematic analysis of prognostic factors for recurrence in a large single institution surgical series. World Neurosurg. 2019;123:e273-e279.
doi pubmed - Bender L, Somme F, Lhermitte B, Ahle G, Boone M, Blonski M, Pouget C, et al. High risk of recurrence for grade II meningioma: a 10-year multicenter analysis of prognosis factors. Chin Clin Oncol. 2021;10(3):26.
doi pubmed - Ong K, Rizzuto M, Makarenko S. Location pattern of recurrence of fully resected grade 1 meningiomas. Acta Neurochir (Wien). 2023;165(10):2865-2871.
doi pubmed - Kawabata S, Kashiwagi H, Nonoguchi N, Furuse M, Takami T, Miyatake SI, Wanibuchi M. [Defining the role of radiotherapy in meningioma treatment]. No Shinkei Geka. 2024;52(4):782-793.
doi pubmed - Wang JZ, Nassiri F, Landry AP, Patil V, Rebchuk A, Merali ZA, Gui C, et al. Fractionated radiotherapy for surgically resected intracranial meningiomas: a multicentre retrospective cohort study. Radiother Oncol. 2023;188:109861.
doi pubmed - Maiuri F, Corvino S, Corazzelli G, Berardinelli J, Di Crescenzo RM, Del Basso De Caro M. Time to recurrence of intracranial meningiomas from a monoinstitutional surgical series. World Neurosurg. 2024;185:e612-e619.
doi pubmed - Lei H, Tabor JK, O'Brien J, Qin R, Pappajohn AF, Chavez MAM, Morales-Valero SF, et al. Associations of race and socioeconomic status with outcomes after intracranial meningioma resection: a systematic review and meta-analysis. J Neurooncol. 2023;163(3):529-539.
doi pubmed - Balik V, Kourilova P, Sulla I, Vrbkova J, Srovnal J, Hajduch M, Takizawa K. Recurrence of surgically treated parasagittal meningiomas: a meta-analysis of risk factors. Acta Neurochir (Wien). 2020;162(9):2165-2176.
doi pubmed - Kim D, Niemierko A, Hwang WL, Stemmer-Rachamimov AO, Curry WT, Barker FG, Martuza RL, et al. Histopathological prognostic factors of recurrence following definitive therapy for atypical and malignant meningiomas. J Neurosurg. 2018;128(4):1123-1132.
doi pubmed - Go KG, Wilmink JT, Molenaar WM. Peritumoral brain edema associated with meningiomas. Neurosurgery. 1988;23(2):175-179.
doi pubmed - Hou J, Kshettry VR, Selman WR, Bambakidis NC. Peritumoral brain edema in intracranial meningiomas: the emergence of vascular endothelial growth factor-directed therapy. Neurosurg Focus. 2013;35(6):E2.
doi pubmed - Berhouma M, Jacquesson T, Jouanneau E, Cotton F. Pathogenesis of peri-tumoral edema in intracranial meningiomas. Neurosurg Rev. 2019;42(1):59-71.
doi pubmed - Gilbert JJ, Paulseth JE, Coates RK, Malott D. Cerebral edema associated with meningiomas. Neurosurgery. 1983;12(6):599-605.
doi pubmed - Javerzat S, Auguste P, Bikfalvi A. The role of fibroblast growth factors in vascular development. Trends Mol Med. 2002;8(10):483-489.
doi pubmed - Bitzer M, Opitz H, Popp J, Morgalla M, Gruber A, Heiss E, Voigt K. Angiogenesis and brain oedema in intracranial meningiomas: influence of vascular endothelial growth factor. Acta Neurochir (Wien). 1998;140(4):333-340.
doi pubmed - Nassehi D. Intracranial meningiomas, the VEGF-A pathway, and peritumoral brain oedema. Dan Med J. 2013;60(4):B4626.
pubmed - Ding YS, Wang HD, Tang K, Hu ZG, Jin W, Yan W. Expression of vascular endothelial growth factor in human meningiomas and peritumoral brain areas. Ann Clin Lab Sci. 2008;38(4):344-351.
pubmed - Rezaee H, Abbasnia S, Alenabi A, Vakili R, Moheghi N, Tavakol Afshari J, Rezaee SA. Expression of vascular endothelial growth factor A and its type 1 receptor in supratentorial neoplasm. Rep Biochem Mol Biol. 2021;10(3):354-361.
doi pubmed - Jabini R, Moradi A, Afsharnezhad S, Ayatollahi H, Behravan J, Raziee HR, Mosaffa F. Pathodiagnostic parameters and evaluation of O(6)- methyl guanine methyl transferase gene promoter methylation in meningiomas. Gene. 2014;538(2):348-353.
doi pubmed - Dharmalingam P, Roopesh Kumar VR, Verma SK. Vascular endothelial growth factor expression and angiogenesis in various grades and subtypes of meningioma. Indian J Pathol Microbiol. 2013;56(4):349-354.
doi pubmed - Ahmeti H, Caliebe A, Rocken C, Jansen O, Mehdorn MH, Synowitz M. Impact of peritumoral brain edema on pre- and postoperative clinical conditions and on long-term outcomes in patients with intracranial meningiomas. Eur J Med Res. 2023;28(1):40.
doi pubmed - Mantle RE, Lach B, Delgado MR, Baeesa S, Belanger G. Predicting the probability of meningioma recurrence based on the quantity of peritumoral brain edema on computerized tomography scanning. J Neurosurg. 1999;91(3):375-383.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.