| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 6, December 2024, pages 902-921
The Prevalence of 5-Fluorouracil and Capecitabine Cardiotoxicity: A Systematic Review and Meta-Analysis
Bannawich Sapapsapa , Poomipat Thongnoia
, Anchana Pongpuna
, Supattra Kitcharoenpanyab
, Teerarat Todsarota
, Arpa Petchsomrita
, Nattawut Leelakanoka, c
aDivision of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Burapha University, Chonburi, Thailand
bFriend Pharmacy, Chonburi, Thailand
cCorresponding Author: Nattawut Leelakanok, Division of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Burapha University, Chonburi 20131, Thailand
Manuscript submitted June 26, 2024, accepted August 13, 2024, published online October 30, 2024
Short title: Cardiotoxicity of 5-FU and Capecitabine
doi: https://doi.org/10.14740/wjon1920
| Abstract | ▴Top |
Background: The incidence of cardiotoxicity events in patients who use 5-fluorouracil (5-FU) and capecitabine monotherapy remains unclear since previous studies reported the prevalence in patients who used combination regimens. We aimed to systematically review and meta-analyze the incidence of cardiotoxicity in fluorouracil and capecitabine monotherapy users.
Methods: The study protocol was registered with PROSPERO (CRD42023441627). Systematic searches were conducted in five databases (CINAHL, OpenGrey, PubMed, ScienceDirect, and Scopus). The Cochrane Risk-of-Bias tool and the Risk Of Bias In Non-randomized Studies were used to evaluate the risk of bias. Pooled prevalence and 95% confidence interval (CI) were calculated using the DerSimonian-Laird random effect models. The funnel plot was used to assess the publication bias.
Results: Eighty studies were included. There were 24 randomized controlled trials (RCTs) with low to high risk of bias and 56 non-RCTs with critical risk of bias. The pooled prevalence of cardiotoxicity from 5-FU was 3.5% (95% CI: 2.7 - 4.2; P < 0.001; I2 = 73.86%). The pooled prevalence of cardiotoxicity in capecitabine users was 2.8% (95% CI: 1.6 - 4.0; P < 0.001; I2 = 72.62%).
Conclusions: The prevalence of cardiotoxicity from 5-FU and capecitabine was classified as common. Cardiotoxicity may have not been associated with the cumulative dose of 5-FU or capecitabine.
Keywords: 5-Fluorouracil; Capecitabine; Cardiotoxicity
| Introduction | ▴Top |
5-Fluorouracil (5-FU) and its oral pro-drug, capecitabine, are anticancer drugs that exert their biological activity by inhibiting DNA synthesis [1]. They are widely used as monotherapy or in combination regimens for the treatment of solid malignancies including colorectal cancer and other types of cancer, e.g., head and neck cancer, esophageal cancer, stomach cancer, and bladder cancer [2]. In addition, they can be combined with radiation therapy or concurrent chemoradiation (CCRT) [3]. 5-FU and capecitabine are significantly crucial for the treatment of cancer that the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology recommend 5-FU and capecitabine as the first-line treatment for metastatic colorectal cancer [4] and the World Health Organization (WHO) listed 5-FU and its oral pro-drug in the 21st list of the WHO Model List of Essential Medicine [5]. However, the use of 5-FU and its pro-drug can be limited by their toxicities.
The dose-limiting toxicities of 5-FU bolus injection are associated with hematological toxicities but 5-FU continuous infusion causes hand-foot syndrome, diarrhea, and mucositis [6, 7]. In addition, cardiac toxicities, which include chest pain, coronary vasospasm, and myocardial infarction, are common for fluorouracil. Other less common symptoms include congestive heart failure, arrhythmias, pericarditis, and sudden cardiac death [8, 9]. Although cardiotoxicity induced by 5-FU has long been discovered [10], and the prevalence is known to be the second most commonly reported after anthracyclines [11], the exact incidence of fluorouracil-induced cardiotoxicity is difficult to estimate. This is because most of the research reported the prevalence or incidence of cardiotoxicity from fluorouracil-containing regimens whose compositions are largely diverse. In addition, most studies fail to report the total number of patients who are treated with fluorouracil which complicates the estimation of such prevalence [12-15].
The Council for International Organizations of Medical Sciences [16] categorized the prevalence of adverse drug reactions (ADRs) into very common, common (or frequent), uncommon (or infrequent), rare, and very rare. The categorization of the risk of cardiotoxicities from 5-FU and capecitabine assists the risk communication to the patients, which affects how patients perceive the risk and adhere to the anticancer treatment [17]. Since the incidence of adverse events is crucial for patient care, we aimed to systematically review and meta-analyze the incidence of cardiotoxicity during the use of fluorouracil.
| Materials and Methods | ▴Top |
Search strategy
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 [18]. The study protocol was registered with PROSPERO (CRD42023441627). The Institutional Review Board (IRB) approval does not apply to this study. Systematic searches were conducted in five databases (CINAHL, OpenGrey, PubMed, ScienceDirect, and Scopus) without language and study design restrictions. The search was conducted from their inception to June 1, 2024. The following search concept was used: “5-fluorouracil” OR “capecitabine” AND “cardiotoxicity” (Supplementary Material 1, wjon.elmerpub.com).
Study eligibility criteria
The systematic review was conducted using the following eligibility criteria: 1) were clinical trials or observational studies; 2) explicitly reported details of 5-FU or capecitabine treatment regiments; 3) used 5-FU or capecitabine as monotherapy for any cancer treatment indication; and 4) explicitly reported the number of cardiotoxicity events. We excluded studies that did not have sufficient data on cardiotoxicity incidence.
Data extraction
Three authors independently screened retrieved articles’ titles, abstracts, and full texts. Disagreements were resolved by consulting the senior author in the team. Also independently, three authors extracted the author’s name, year of publication, region, study design, sample size, duration of the study, mean age, sex, characteristics of participants, comorbidity of participants, cancer type, treatment regimen, cumulative dose, and cardiotoxicity events. Data deemed important but unavailable in the publications were retrieved by contacting the corresponding authors. The articles were excluded if the corresponding authors did not respond in a reasonable time. The median age (with interquartile range) was converted to mean and standard deviation using a formula by Wan et al [19]. The cumulative dose was calculated from the total 5-FU or capecitabine dose received before cardiotoxicity occurred. ADRs were classified according to the Council for International Organizations of Medical Sciences [16] into five groups as follows: very common (frequency higher than 1/10), common (less than 1/10 to higher than 1/100), uncommon (less than 1/100 to higher than 1/1,000), rare (less than 1/1,000 to higher than 1/10,000), and very rare (less than 1/10,000).
Quality assessment
Three authors independently assessed the risk of bias in the included studies. The risk of bias in randomized controlled trials and non-randomized trials was evaluated using the Cochrane Risk-of-Bias tool 2.0 (RoB 2.0) [20], and the Risk Of Bias In Non-randomized Studies (ROBINs) [21], respectively.
Statistical analysis
The pooled prevalence of cardiotoxicity induced by 5-FU or capecitabine and 95% confidence interval (CI) were calculated using the DerSimonian-Laird random effect models [22] (OpenMetaAnalyst for Windows 8). Heterogeneity was assessed using Cochrane’s Q statistic and I2 values. The P-value of Cochrane’s Q of less than 0.10 was considered significant. I2 of greater than 75% indicated high heterogeneity while I2 of less than 25% indicated high homogeneity [23]. The funnel plot was used to observe publication bias.
Subgroup analysis and meta-regression
The influence of baseline characteristics, which may have caused heterogeneity, was determined by meta-regression (OpenMetaAnalyst for Windows 8 [24]). The effect of the following variables was planned for the analysis a priori: age, sex, study site, total cumulative dose, and year of study.
| Results | ▴Top |
Study characteristics
From 34,705 articles from the systematic search, 80 studies were selected for meta-analysis [8, 15, 25-102]. Twenty-four were randomized studies and 56 were non-randomized studies. Details of the systematic search and screening are shown in the PRISMA diagram (Fig. 1). This systematic review included 18,524 participants, 5,836 of which were males. The average age of the participants was approximately 53.65 ± 7.32 years old. Most had gastrointestinal cancer such as colorectal cancer or gastric cancer. Almost all studies did not report patients’ medications for other comorbidities. Baseline characteristics are shown in Table 1 and additional participant characteristics are in Supplementary Material 2 (wjon.elmerpub.com). Information on dosage regimens and cardiotoxicity outcomes in patients who received 5-FU or capecitabine monotherapy is shown in Supplementary Materials 3 and 4 (wjon.elmerpub.com).
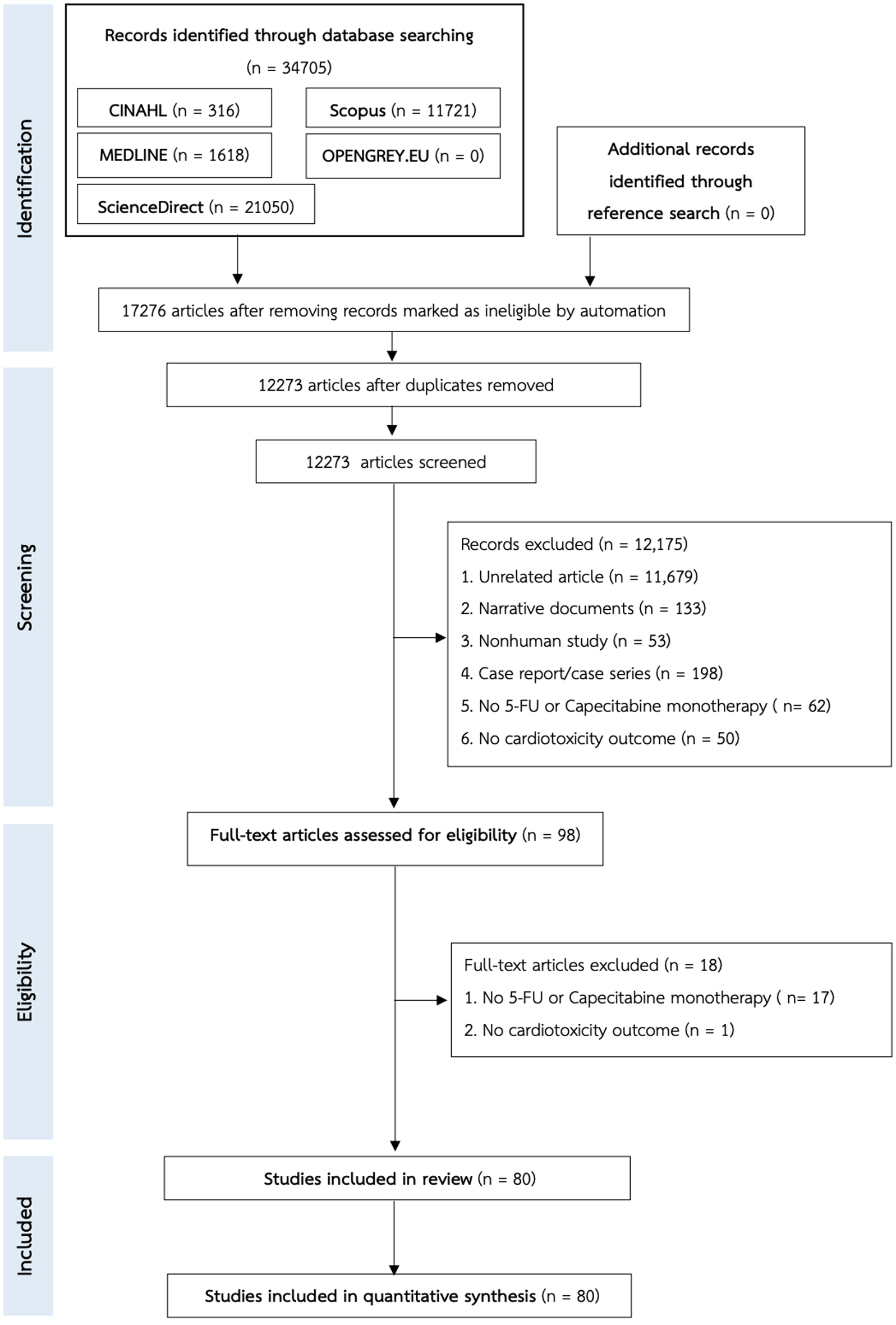 Click for large image | Figure 1. The PRISMA flow chart of the study selection. |
 Click to view | Table 1. Characteristics of the Included Studies |
Risk of bias assessment
We found two randomized studies with a high risk of bias [27, 57] since more than 10% of data were missed and reasons for missing patients were not reported. The remaining had some concerns (N = 17) and a low risk of bias (N = 5) (Fig. 2). All non-randomized studies (N = 56) reported a crude prevalence of cardiotoxicity outcomes, so they were evaluated as having a critical risk of bias (Fig. 3).
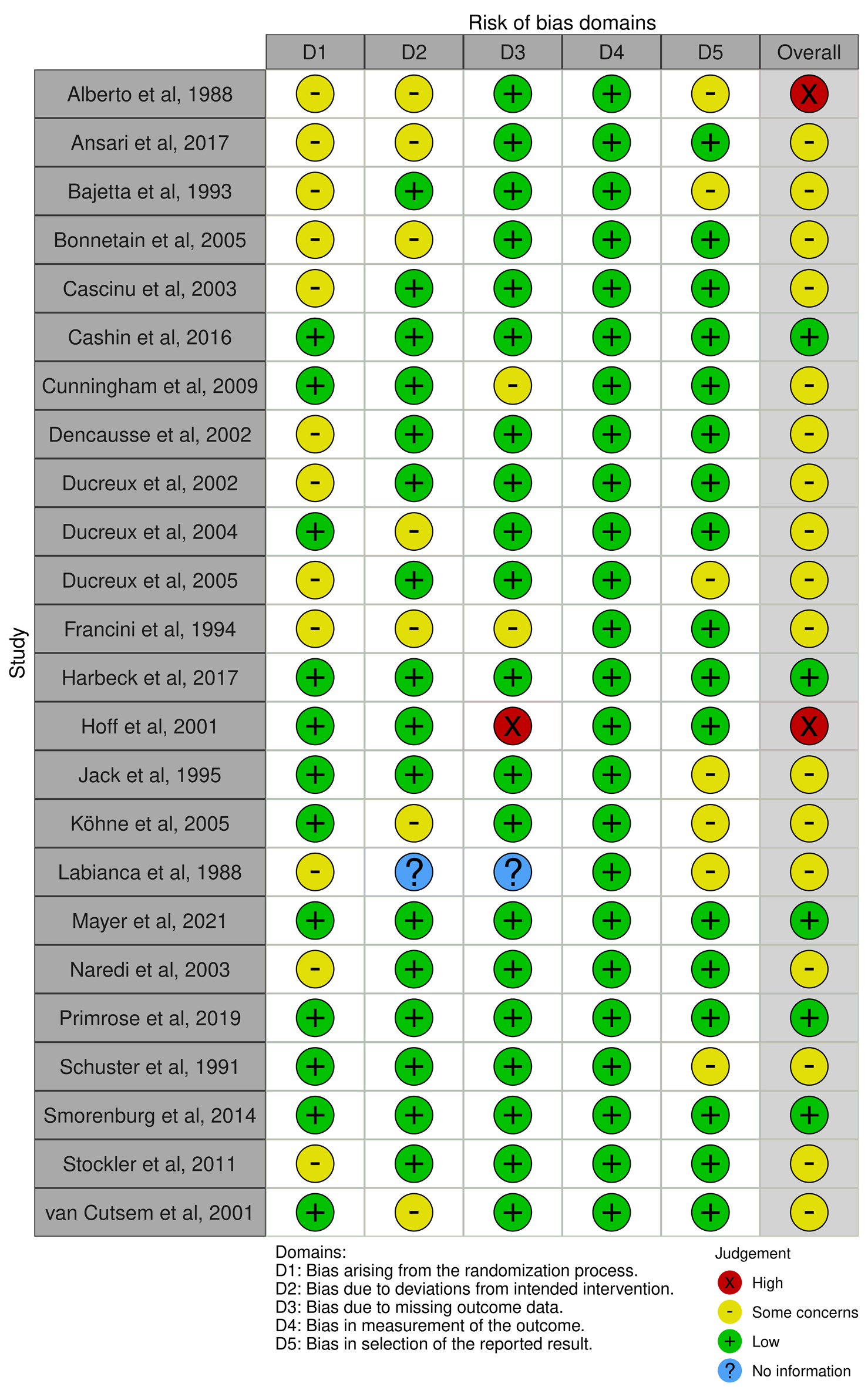 Click for large image | Figure 2. The evaluation of the risk of bias in randomized control trials using the Cochrane Risk-of-Bias tool 2.0 (RoB 2.0). |
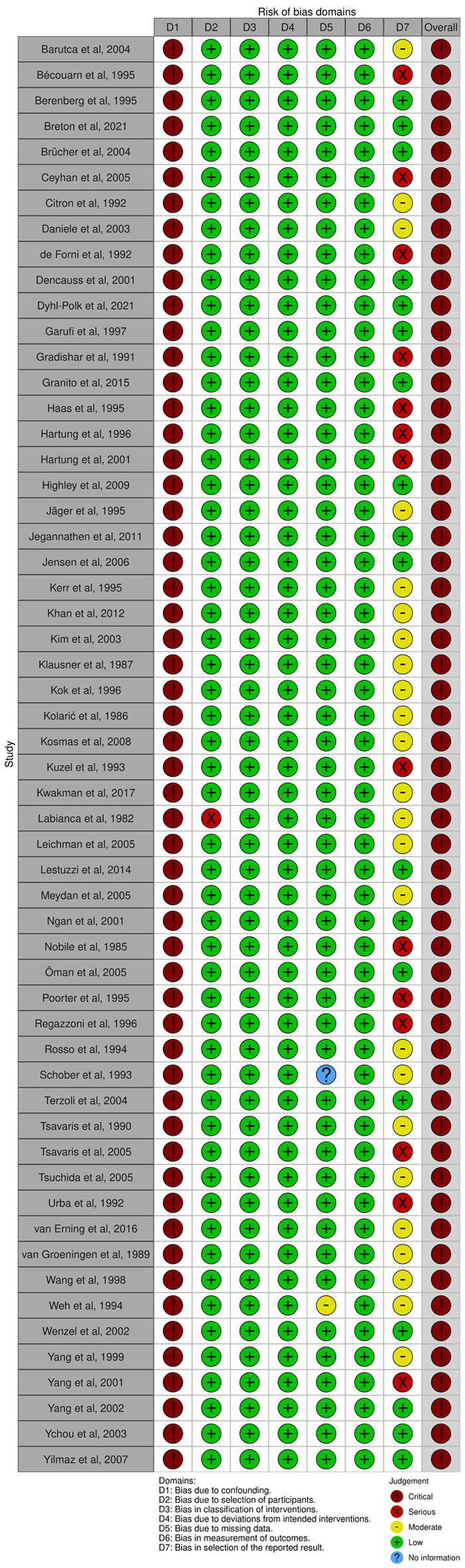 Click for large image | Figure 3. The evaluation of the risk of bias in non-randomized studies using The Risk Of Bias In Non-randomized Studies (ROBINs). |
Prevalence of 5-FU and capecitabine cardiotoxicity
Of 80 included studies, 70 reported the prevalence of cardiotoxicity in 5-FU users. The pooled prevalence of cardiotoxicity was 3.5% (95% CI: 2.7 - 4.2; P < 0.001; I2 = 73.86%; Fig. 4). In addition, 14 studies reported the prevalence of cardiotoxicity in capecitabine users. The pooled prevalence of cardiotoxicity was 2.8% (95% CI: 1.6 - 4.0; P < 0.001; I2 = 72.62%; Fig. 5). Cardiotoxicity from 5-FU and capecitabine was classified as common by the Council for International Organizations of Medical Sciences criteria. The funnel plot showed that most included studies were small. The symmetry was not evaluable since the pooled prevalence was close to zero causing the plot to distribute at the positive side of the funnel (Fig. 6).
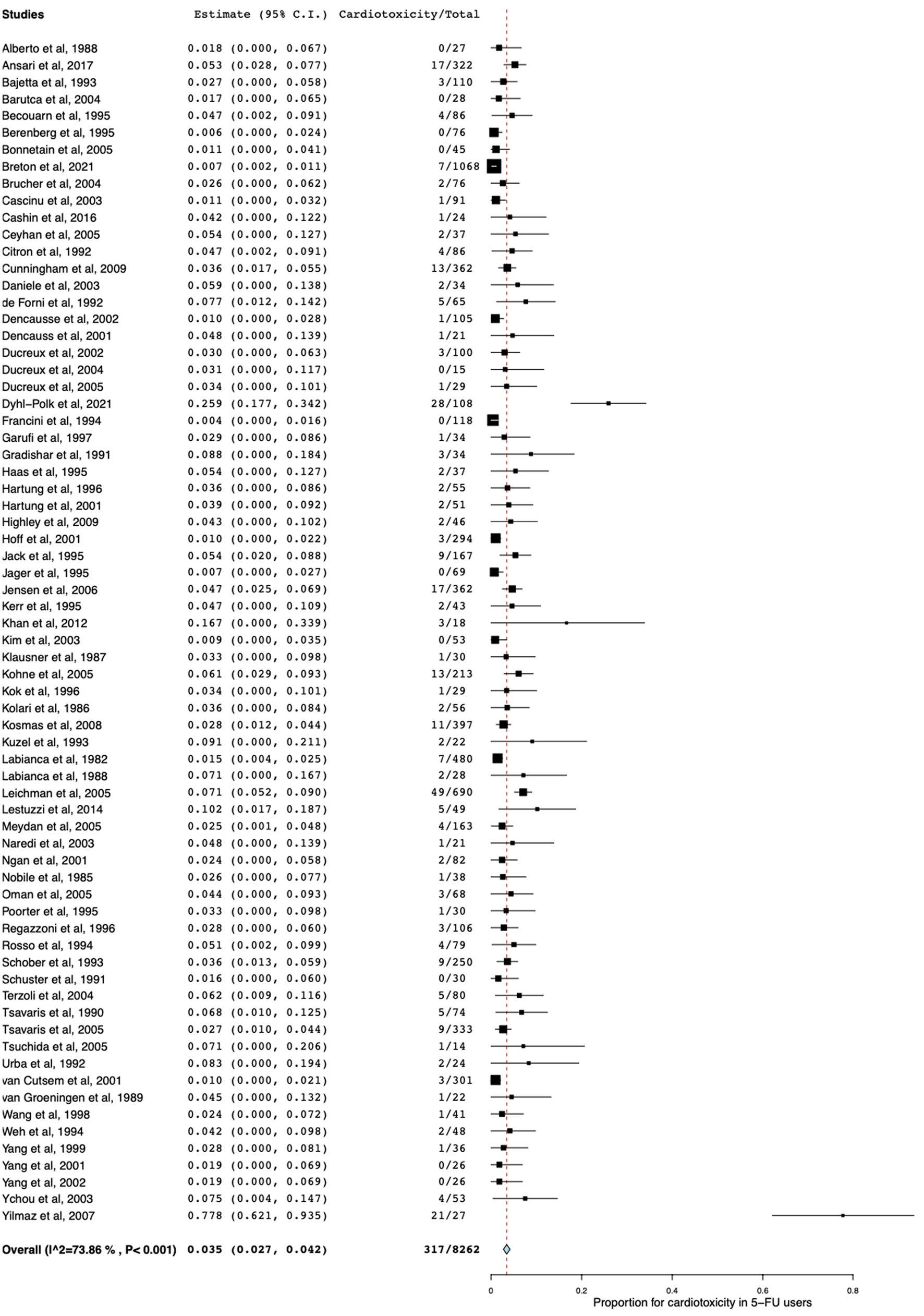 Click for large image | Figure 4. Forest plot of cardiotoxicity in patients receiving 5-FU monotherapy. 5-FU: 5-fluorouracil. |
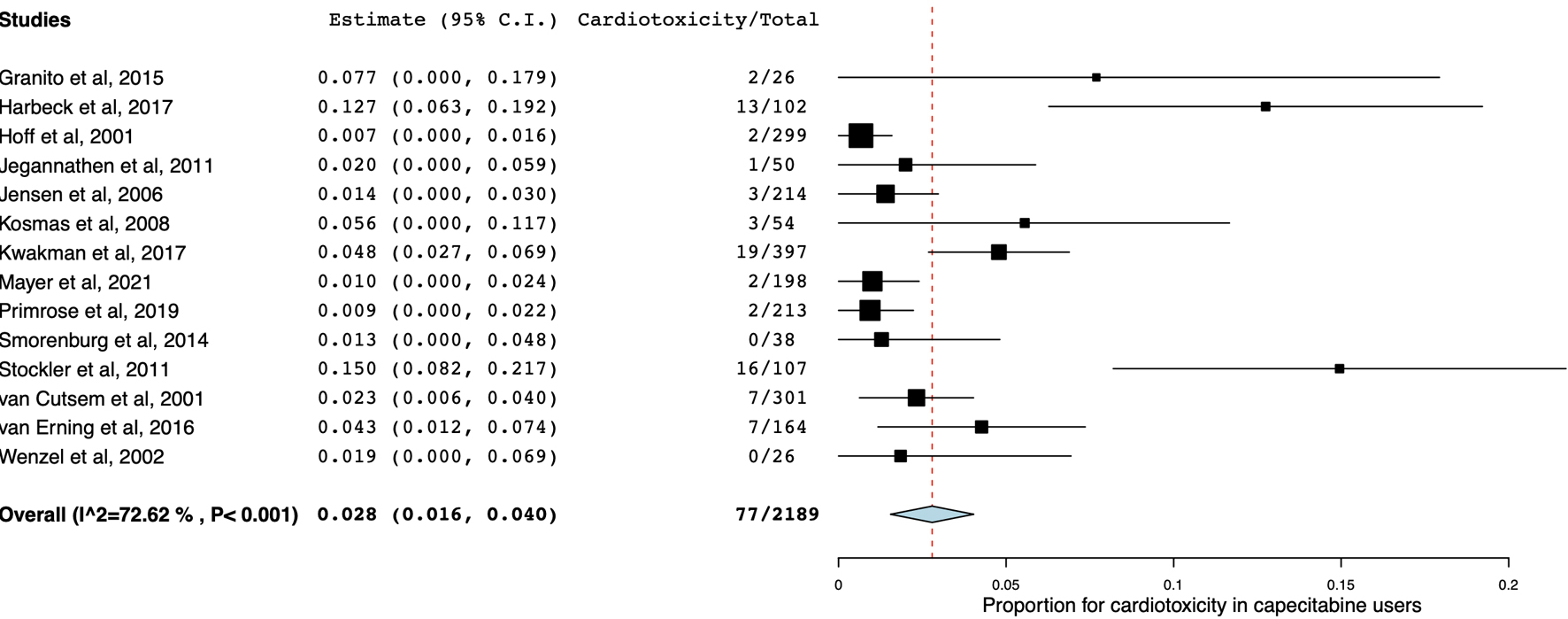 Click for large image | Figure 5. Forest plot of cardiotoxicity in patients receiving capecitabine monotherapy. |
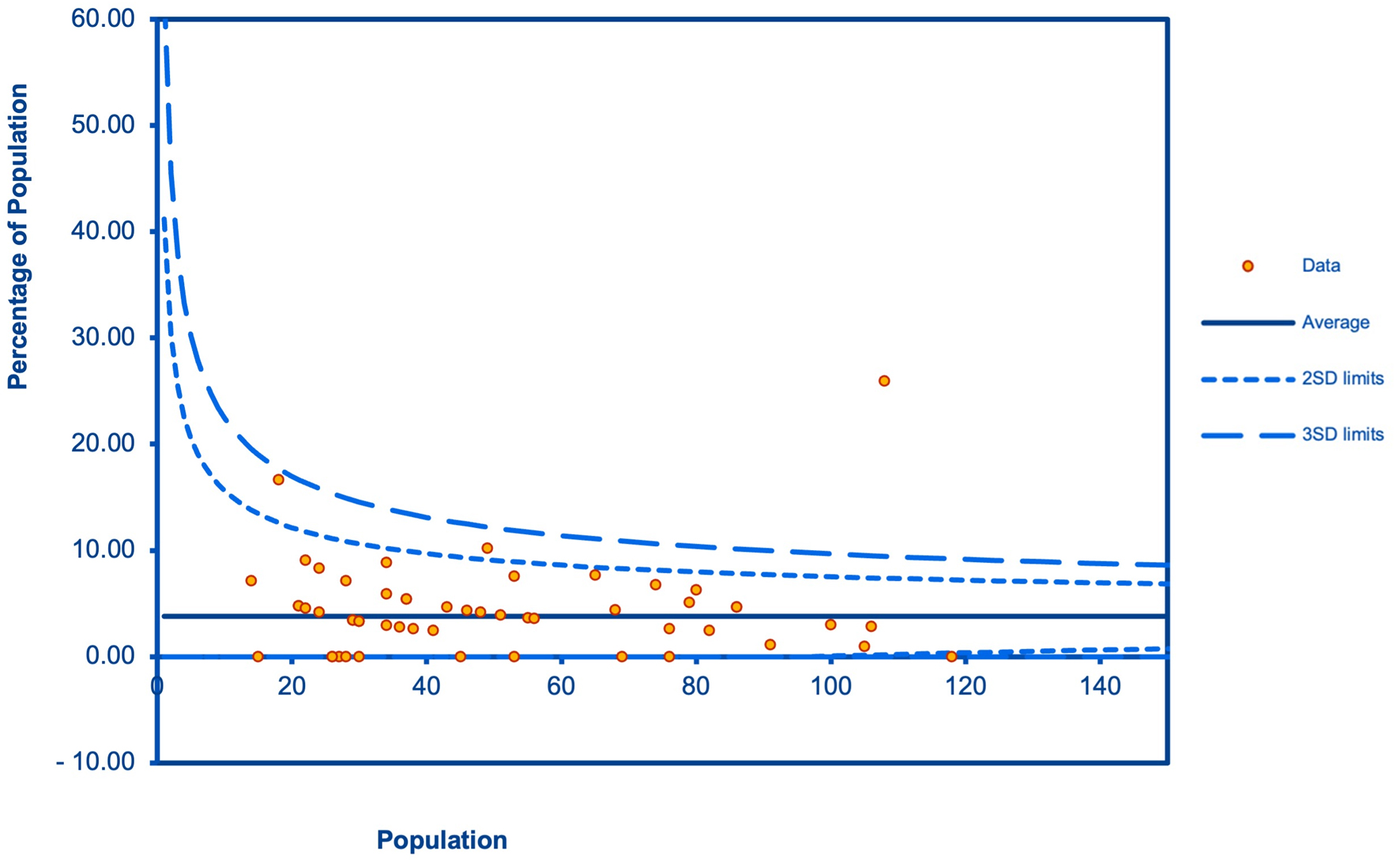 Click for large image | Figure 6. A funnel plot for the publication bias. |
Meta-regression
Meta-regression revealed that the heterogeneity in the analysis of cardiotoxicity in 5-FU users was not caused by age, cumulative dose, place of study, sex, and year of study (P-value ≥ 0.099 in all analyses). The heterogeneity in the analysis of cardiotoxicity in capecitabine users was caused by place of study (P-value = 0.001) but not age, cumulative dose, sex, and year of study (P-value > 0.1 in all analyses). Details of the meta-regression analysis are shown in Supplementary Materials 5 and 6 (wjon.elmerpub.com).
| Discussion | ▴Top |
The prevalence of any cardiotoxic events in 5-FU users was 3.5% and 2.8% in capecitabine users. Although cardiotoxicity was classified as common, the prevalence was still lower than other common ADRs. For example, the most common ADRs to 5-FU were diarrhea (64%), stomatitis (60%), and nausea/vomiting (51%), while the most common ADRs to capecitabine were hand-foot syndrome (62%), diarrhea (46%), and nausea/vomiting (36%) [103]. The prevalence of cardiotoxicity from 5-FU and capecitabine was lower than drugs well-known for cardiotoxicity such as anthracyclines, cyclophosphamide, and docetaxel which have a prevalence of, approximately 9% [104], 7-28%, and 2.3-8% [105], respectively. While our meta-analysis reported the estimated prevalence of cardiotoxicity from fluoropyrimidine monotherapy regimen as approximately 3%, a systematic review of cardiotoxicity from 5-FU and capecitabine as either monotherapy or combination regimens reports the prevalence as 0-20% and 3-35%, respectively [106].
Cardiotoxicity from fluoropyrimidines may be considered type A since evidence supporting pharmacological mechanisms is available. First, the most recognized mechanism is that fluoropyrimidines induce the release of vasoconstrictive mediators. For example, kinase C causes endothelium-independent vasoconstriction [107], and endothelin-1 is a potent vasoconstrictor that can induce coronary artery disease [108, 109]. Second, fluoropyrimidines induce vascular endothelial dysfunction and impaired oxygen delivery. Animal studies have shown that 5-FU can have direct toxic effects on vascular endothelial cells. This results in direct endothelial damage, fibrin, and platelet accumulation. Additionally, studies have shown that 5-FU can alter erythrocyte membranes, resulting in decreased oxygen transport in the blood and myocardial ischemia [110, 111]. Third, fluoropyrimidines degrade to alpha-fluoro-beta-alanine (FBAL) which causes a direct toxic effect on cardiomyocytes [112, 113]. From these mechanisms, clinical symptoms from the most common cardiotoxicity are angina and the less common are arrhythmias, myocardial infarction, heart failure, acute pulmonary edema, and cardiac arrest [114].
The cardiotoxicity of 5-FU and capecitabine can occur in the first cycle of use, 12 - 48 h after receiving the first dose [115]. The risk factors are not well understood. For example, the effect of pre-existing cardiovascular diseases [8, 25, 26, 116] on cardiotoxicity is inconclusive. Anyhow, there are some limitations in our study. First, our study analyzed the data from fluoropyrimidine monotherapy only. This may not reflect the cardiotoxicity in patients who used combination regimens with fluoropyrimidine and other drugs. Second, the risk of bias in the included studies was high. This is expected since prevalent studies are highly affected by biases in nature. Third, most studies did not report the severity of cardiotoxicity so the authors could not incorporate the severity data in the meta-analysis. Next, we cannot distinguish the cardiotoxicity from bolus versus continuous 5-FU since most studies used both types of administration. Last, we included seven studies [15, 26, 41, 47, 52, 74, 83] whose minor fraction of participants had pre-existing cardiac conditions. These studies did not specify whether the cardiotoxicity occurred in patients with pre-existing cardiac conditions. In addition, the small number of studies discouraged the meta-regression to determine the effect of pre-existing cardiac conditions on the pooled estimate.
This is the first systematic review and meta-analysis that identifies the prevalence of cardiotoxicity in 5-FU and capecitabine monotherapy users. The number of included participants is large so the prevalence can be reported more accurately. There are several applications for this study. First, although cardiotoxicity from fluoropyrimidines may be dose-dependent, this study did not support the association between the cumulative dose of 5-FU or capecitabine and the cardiotoxicity prevalence. The management of cardiotoxicity should be based on how type B reactions are managed including discontinuation. Non-dihydropyridine calcium channel blockers and nitrates should also be provided [114]. Second, cardiotoxicity is common and can include serious events. Therefore, patients should be followed up from the first cycle until the end of treatment. The follow-up should include an electrocardiography (EKG) which allows the detection of subclinical cardiotoxicity. Patients with underlying heart diseases should be closely monitored. Some studies suggest that colorectal cancer patients with dihydropyrimidine dehydrogenase (DPD) deficiency have an increased risk of cardiotoxicity, and therefore pretreatment screening of DPD activity may be considered [117]. Future studies should include studies that evaluate risk factors for cardiotoxicity from 5-FU or capecitabine more accurately.
Conclusion
The prevalence of cardiotoxicity from 5-FU and capecitabine was 3.5% and 2.8%, respectively. We did not find evidence that cardiotoxicity was associated with the cumulative dose of 5-FU or capecitabine.
| Supplementary Material | ▴Top |
Suppl 1. Search term.
Suppl 2. Additional baseline characteristics of the included studies.
Suppl 3. Details of the dosage regimen and cardiotoxicity outcomes in patients who received 5-FU monotherapy.
Suppl 4. Details of the dosage regimen and cardiotoxicity outcomes in patients who received capecitabine monotherapy.
Suppl 5. Meta-regression for an association between the cardiotoxicity prevalence in 5-FU users and demographic data (age, cumulative dose, place of study, sex, and year of study).
Suppl 6. Meta-regression for an association between the cardiotoxicity prevalence in capecitabine users and demographic data (age, cumulative dose, place of study, sex, and year of study).
Acknowledgments
None to declare.
Financial Disclosure
This work was financially supported by the Research Grant of the Faculty of Pharmaceutical Science, Burapha University (grant no. 2/2566 (extra)).
Conflict of Interest
There is nothing to declare.
Informed Consent
Not applicable.
Author Contributions
BS and NL contributed to the research idea and design. BS, PT, AnP, SK, and TT contributed to data collection. BS and NL contributed to the statistical analysis and interpretation of data. BS wrote the first draft of the manuscript. NL and ArP edited the draft of the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content and approved and reviewed the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ADRs: adverse drug reactions; 95% CI: 95% confidence interval; CCRT: concurrent chemoradiation; EKG: electrocardiography; FBAL: alpha-fluoro-beta-alanine; 5-FU: 5-fluorouracil; NCCN: National Comprehensive Cancer Network; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RoB 2.0: the Cochrane Risk-of-Bias tool 2.0; ROBINs: the Risk Of Bias In Non-randomized Studies; WHO: World Health Organization
| References | ▴Top |
- Myers CE. The pharmacology of the fluoropyrimidines. Pharmacol Rev. 1981;33(1):1-15.
pubmed - Grem JL. 5-Fluorouracil: forty-plus and still ticking. A review of its preclinical and clinical development. Invest New Drugs. 2000;18(4):299-313.
doi pubmed - Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, Peiffert D, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15(5):2040-2049.
doi pubmed - National Comprehensive Cancer Network. Colon Cancer (version 2.2023). 2022. Retrieved from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- World Health Organization. WHO model list of essential medicines - 22nd list. 2021. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02. Accessed June 29, 2023.
- Meta-analysis Group In C, Piedbois P, Rougier P, Buyse M, Pignon J, Ryan L, Hansen R, et al. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16(1):301-308.
doi pubmed - Malet-Martino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. Oncologist. 2002;7(4):288-323.
doi pubmed - de Forni M, Malet-Martino MC, Jaillais P, Shubinski RE, Bachaud JM, Lemaire L, Canal P, et al. Cardiotoxicity of high-dose continuous infusion fluorouracil: a prospective clinical study. J Clin Oncol. 1992;10(11):1795-1801.
doi pubmed - Sorrentino MF, Kim J, Foderaro AE, Truesdell AG. 5-fluorouracil induced cardiotoxicity: review of the literature. Cardiol J. 2012;19(5):453-458.
doi pubmed - Gaveau T, Banzet P, Marneffe H, Viars P. [Cardiovascular disorders in the course of antimitotic infusions at high doses. 30 clinical cases]. Anesth Analg (Paris). 1969;26(3):311-327.
pubmed - Anand AJ. Fluorouracil cardiotoxicity. Ann Pharmacother. 1994;28(3):374-378.
doi pubmed - Meyer CC, Calis KA, Burke LB, Walawander CA, Grasela TH. Symptomatic cardiotoxicity associated with 5-fluorouracil. Pharmacotherapy. 1997;17(4):729-736.
pubmed - Wacker A, Lersch C, Scherpinski U, Reindl L, Seyfarth M. High incidence of angina pectoris in patients treated with 5-fluorouracil. A planned surveillance study with 102 patients. Oncology. 2003;65(2):108-112.
doi pubmed - Holubec L, Jr., Topolcan O, Finek J, Salvet J, Svoboda T, Svobodova S, Mrazkova P, et al. Dynamic monitoring of cardio-specific markers and markers of thyroid gland function in cancer patients—a pilot study. Anticancer Res. 2007;27(4A):1883-1886.
pubmed - Lestuzzi C, Vaccher E, Talamini R, Lleshi A, Meneguzzo N, Viel E, Scalone S, et al. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: an underestimated risk. Ann Oncol. 2014;25(5):1059-1064.
doi pubmed - Reactions Reporting Adverse Drug. Definitions of terms and criteria for their use. Geneva: World Health Organization; 1999.
- Buchter RB, Fechtelpeter D, Knelangen M, Ehrlich M, Waltering A. Words or numbers? Communicating risk of adverse effects in written consumer health information: a systematic review and meta-analysis. BMC Med Inform Decis Mak. 2014;14:76.
doi pubmed pmc - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed pmc - Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
doi pubmed pmc - Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
doi pubmed - Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
doi pubmed pmc - DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
doi pubmed - Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560.
doi pubmed pmc - Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80.
doi pubmed pmc - Jensen SA, Sorensen JB. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol. 2006;58(4):487-493.
doi pubmed - Labianca R, Beretta G, Clerici M, Fraschini P, Luporini G. Cardiac toxicity of 5-fluorouracil: a study on 1083 patients. Tumori. 1982;68(6):505-510.
doi pubmed - Alberto P, Mermillod B, Germano G, Kaplan S, Weber W, Joss R, Spati B, et al. A randomized comparison of doxifluridine and fluorouracil in colorectal carcinoma. Eur J Cancer Clin Oncol. 1988;24(3):559-563.
doi pubmed - Ansari N, Solomon MJ, Fisher RJ, Mackay J, Burmeister B, Ackland S, Heriot A, et al. Acute adverse events and postoperative complications in a randomized trial of preoperative short-course radiotherapy versus long-course chemoradiotherapy for T3 adenocarcinoma of the rectum: trans-tasman radiation oncology group trial (TROG 01.04). Ann Surg. 2017;265(5):882-888.
doi pubmed - Bajetta E, Colleoni M, Rosso R, Sobrero A, Amadori D, Comella G, Marangolo M, et al. Prospective randomised trial comparing fluorouracil versus doxifluridine for the treatment of advanced colorectal cancer. Eur J Cancer. 1993;29A(12):1658-1663.
doi pubmed - Barutca S, Ceyhan C, Meydan N, Ozturk B, Tekten T, Onbasili A, Kadikoylu G, et al. A new perspective on cardiotoxicity of 5-fluorouracil. A novel research tool 'cardiac ultrasonic integrated backscatter analysis' indicates transient, subclinical myocardial dysfunction due to high-dose leucovorin and infusional 5-fluorouracil regimen. Chemotherapy. 2004;50(3):113-118.
doi pubmed - Becouarn YH, Brunet RC, Rouhier ML, Bussieres EJ, Avril AR, Richaud PM, Dilhuydy JM. High dose folinic acid and 5-fluorouracil bolus and continuous infusion for patients with advanced colorectal cancer. Cancer. 1995;76(7):1126-1131.
doi pubmed - Berenberg JL, Tangen C, Macdonald JS, Hutchins LF, Natale RB, Oishi N, Guy JT, et al. Phase II study of 5-fluorouracil and folinic acid in the treatment of patients with advanced gastric cancer. A Southwest Oncology Group study. Cancer. 1995;76(5):715-719.
doi pubmed - Bonnetain F, Bouche O, Conroy T, Arveux P, Raoul JL, Giovannini M, Etienne PL, et al. Longitudinal quality of life study in patients with metastatic gastric cancer. Analysis modalities and clinical applicability of QoL in randomized phase II trial in a digestive oncology. Gastroenterol Clin Biol. 2005;29(11):1113-1124.
doi pubmed - Breton C, Aparicio T, Le Malicot K, Ducreux M, Lecomte T, Bachet JB, Taieb J, et al. Predictive factors of severe early treatment-related toxicity in patients receiving first-line treatment for metastatic colorectal cancer: Pooled analysis of 2190 patients enrolled in Federation Francophone de Cancerologie Digestive (FFCD) trials. Eur J Cancer. 2021;153:40-50.
doi pubmed - Brucher BL, Stein HJ, Zimmermann F, Werner M, Sarbia M, Busch R, Dittler HJ, et al. Responders benefit from neoadjuvant radiochemotherapy in esophageal squamous cell carcinoma: results of a prospective phase-II trial. Eur J Surg Oncol. 2004;30(9):963-971.
doi pubmed - Cascinu S, Catalano V, Piga A, Mattioli R, Marcellini M, Pancotti A, Bascioni R, et al. The role of levamisole in the adjuvant treatment of stage III colon cancer patients: a randomized trial of 5-fluorouracil and levamisole versus 5-fluorouracil alone. Cancer Invest. 2003;21(5):701-707.
doi pubmed - Cashin PH, Mahteme H, Spang N, Syk I, Frodin JE, Torkzad M, Glimelius B, et al. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: A randomised trial. Eur J Cancer. 2016;53:155-162.
doi pubmed - Ceyhan C, Meydan N, Barutca S, Tekten T, Onbasili AO, Ozturk B, Unal S. Ultrasound tissue characterization by integrated backscatter for analyzing Fluorouracil induced myocardial damage. Echocardiography. 2005;22(3):233-238.
doi pubmed - Citron ML, Modeas C, Propert K, Goutsou M, Green MR. Phase II trial of high-dose 24-hour continuous intravenous 5-fluorouracil for advanced non-small cell lung cancer: a Cancer and Leukemia Group B study. Cancer Invest. 1992;10(3):215-219.
doi pubmed - Cunningham D, Sirohi B, Pluzanska A, Utracka-Hutka B, Zaluski J, Glynne-Jones R, Koralewski P, et al. Two different first-line 5-fluorouracil regimens with or without oxaliplatin in patients with metastatic colorectal cancer. Ann Oncol. 2009;20(2):244-250.
doi pubmed - Daniele B, Rosati G, Tambaro R, Ottaiano A, De Maio E, Pignata S, Iaffaioli RV, et al. First-line chemotherapy with fluorouracil and folinic acid for advanced colorectal cancer in elderly patients: a phase II study. J Clin Gastroenterol. 2003;36(3):228-233.
doi pubmed - Dencausse Y, Hartung G, Sturm J, Kopp-Schneider A, Hagmuller E, Wojatschek C, Lindemann H, et al. Adjuvant chemotherapy in stage III colon cancer with 5-fluorouracil and levamisole versus 5-fluorouracil and leucovorin. Onkologie. 2002;25(5):426-430.
doi pubmed - Dencausse Y, Sturm J, Hartung G, Dietzler P, Edler L, Bambach M, Wojatschek C, et al. Adjuvant radio-chemotherapy in stage II-III rectal cancer with 24-hour infusion of high-dose 5-fluorouracil and folinic acid: evaluation of feasibility. Onkologie. 2001;24(5):476-480.
doi pubmed - Ducreux M, Rougier P, Pignon JP, Douillard JY, Seitz JF, Bugat R, Bosset JF, et al. A randomised trial comparing 5-FU with 5-FU plus cisplatin in advanced pancreatic carcinoma. Ann Oncol. 2002;13(8):1185-1191.
doi pubmed - Ducreux M, Mitry E, Ould-Kaci M, Boige V, Seitz JF, Bugat R, Breau JL, et al. Randomized phase II study evaluating oxaliplatin alone, oxaliplatin combined with infusional 5-FU, and infusional 5-FU alone in advanced pancreatic carcinoma patients. Ann Oncol. 2004;15(3):467-473.
doi pubmed - Ducreux M, Van Cutsem E, Van Laethem JL, Gress TM, Jeziorski K, Rougier P, Wagener T, et al. A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer. 2005;41(3):398-403.
doi pubmed - Dyhl-Polk A, Schou M, Vistisen KK, Sillesen AS, Serup-Hansen E, Faber J, Klausen TW, et al. Myocardial Ischemia Induced by 5-Fluorouracil: A Prospective Electrocardiographic and Cardiac Biomarker Study. Oncologist. 2021;26(3):e403-e413.
doi pubmed pmc - Francini G, Petrioli R, Lorenzini L, Mancini S, Armenio S, Tanzini G, Marsili S, et al. Folinic acid and 5-fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology. 1994;106(4):899-906.
doi pubmed - Garufi C, Levi F, Aschelter AM, Pace R, Giunta S, Nistico C, Galla DA, et al. A phase I trial of 5-day chronomodulated infusion of 5-fluorouracil and 1-folinic acid in patients with metastatic colorectal cancer. Eur J Cancer. 1997;33(10):1566-1571.
doi pubmed - Gradishar W, Vokes E, Schilsky R, Weichselbaum R, Panje W. Vascular events in patients receiving high-dose infusional 5-fluorouracil-based chemotherapy: the University of Chicago experience. Med Pediatr Oncol. 1991;19(1):8-15.
doi pubmed - Granito A, Marinelli S, Terzi E, Piscaglia F, Renzulli M, Venerandi L, Benevento F, et al. Metronomic capecitabine as second-line treatment in hepatocellular carcinoma after sorafenib failure. Dig Liver Dis. 2015;47(6):518-522.
doi pubmed - Haas NB, Schilder RJ, Nash S, Weiner LM, Catalano RC, Ozols RF, O'Dwyer PJ. A phase II trial of weekly infusional 5-fluorouracil in combination with low-dose leucovorin in patients with advanced colorectal cancer. Invest New Drugs. 1995;13(3):229-233.
doi pubmed - Harbeck N, Saupe S, Jager E, Schmidt M, Kreienberg R, Muller L, Otremba BJ, et al. A randomized phase III study evaluating pegylated liposomal doxorubicin versus capecitabine as first-line therapy for metastatic breast cancer: results of the PELICAN study. Breast Cancer Res Treat. 2017;161(1):63-72.
doi pubmed pmc - Hartung G, Queiber W, Diezler P, Hagmuller E, Edler L, Jacob I, et al. Adjuvant chemotherapy with 5-fluorouracil and folinic acid in colorectal cancer: evaluation of toxicity. Onkologie. 1996;19(1):62-67.
- Hartung G, Hofheinz RD, Wein A, Riedel C, Rost A, Fritze D, Kreuser ED, et al. Phase II study of a weekly 24-hour infusion with 5-fluorouracil and simultaneous sodium-folinic acid in the first-line treatment of metastatic colorectal cancer. Onkologie. 2001;24(5):457-462.
doi pubmed - Highley MS, Griffiths GO, Uscinska BM, Huddart RA, Barber JB, Parmar MK, Harper PG, et al. A phase II trial of continuous 5-fluorouracil in recurrent or metastatic transitional cell carcinoma of the urinary tract. Clin Oncol (R Coll Radiol). 2009;21(5):394-400.
doi pubmed - Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, Maroun J, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19(8):2282-2292.
doi pubmed - Jack WJ, Everington D, Rodger A, Forrest AP, Stewart HJ. Adjuvant therapy with 5-fluorouracil for breast cancer of likely poor prognosis: 15-year results of a randomized trial. Clin Oncol (R Coll Radiol). 1995;7(1):7-11.
doi pubmed - Jager E, Klein O, Wachter B, Muller B, Braun U, Knuth A. Second-line treatment with high-dose 5-fluorouracil and folinic acid in advanced colorectal cancer refractory to standard-dose 5-fluorouracil treatment. Oncology. 1995;52(6):470-473.
doi pubmed - Jegannathen A, Mais K, Sykes A, Lee L, Yap B, Birzgalis A, Homer J, et al. Synchronous chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck using capecitabine: a single-centre, open-label, single-group phase II study. Clin Oncol (R Coll Radiol). 2011;23(2):149-158.
doi pubmed - Kerr DJ, Ledermann JA, McArdle CS, Buckels J, Neoptolemos J, Seymour M, Doughty J, et al. Phase I clinical and pharmacokinetic study of leucovorin and infusional hepatic arterial fluorouracil. J Clin Oncol. 1995;13(12):2968-2972.
doi pubmed - Khan MA, Masood N, Husain N, Ahmad B, Aziz T, Naeem A. A retrospective study of cardiotoxicities induced by 5-fluouracil (5-FU) and 5-FU based chemotherapy regimens in Pakistani adult cancer patients at Shaukat Khanum Memorial Cancer Hospital & Research Center. J Pak Med Assoc. 2012;62(5):430-434.
pubmed - Kim DJ, Kim TI, Suh JH, Cho YS, Shin SK, Kang JK, Kim NK, et al. Oral tegafur-uracil plus folinic acid versus intravenous 5-fluorouracil plus folinic acid as adjuvant chemotherapy of colon cancer. Yonsei Med J. 2003;44(4):665-675.
doi pubmed - Klausner JM, Gutman M, Rozin RR, Lelcuk S, Chaitchik S, Inbar M. Conventional fractionation radiotherapy combined with 5-fluorouracil for metastatic malignant melanoma. Am J Clin Oncol. 1987;10(5):448-450.
doi pubmed - Kohne CH, van Cutsem E, Wils J, Bokemeyer C, El-Serafi M, Lutz MP, Lorenz M, et al. Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J Clin Oncol. 2005;23(22):4856-4865.
doi pubmed - Kok TC, van der Gaast A, Splinter TA. 5-fluorouracil and folinic acid in advanced adenocarcinoma of the esophagus or esophago-gastric junction area. Rotterdam Esophageal Tumor Study Group. Ann Oncol. 1996;7(5):533-534.
doi pubmed - Kolaric K, Potrebica V, Stanovnik M. Controlled phase III clinical study of 4-epi-doxorubicin + 5-fluorouracil versus 5-fluorouracil alone in metastatic gastric and rectosigmoid cancer. Oncology. 1986;43(2):73-77.
doi pubmed - Kosmas C, Kallistratos MS, Kopterides P, Syrios J, Skopelitis H, Mylonakis N, Karabelis A, et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol. 2008;134(1):75-82.
doi pubmed - Kuzel TM, Tallman MS, Shevrin D, Braud E, Kilton L, Johnson P, Kozlowski J, et al. A phase II study of continuous infusion 5-fluorouracil in advanced hormone refractory prostate cancer. An Illinois Cancer Center Study. Cancer. 1993;72(6):1965-1968.
doi pubmed - Kwakman JJ, Simkens LH, Mol L, Kok WE, Koopman M, Punt CJ. Incidence of capecitabine-related cardiotoxicity in different treatment schedules of metastatic colorectal cancer: a retrospective analysis of the CAIRO studies of the Dutch Colorectal Cancer Group. Eur J Cancer. 2017;76:93-99.
doi pubmed - Labianca R, Pancera G, Cesana B, Clerici M, Montinari F, Luporini G. Cisplatin + 5-fluorouracil versus 5-fluorouracil alone in advanced colorectal cancer: a randomized study. Eur J Cancer Clin Oncol. 1988;24(10):1579-1581.
doi pubmed - Leichman CG, Benedetti JK, Zalupski MM, Hochster H, Shields AF, Lenz HJ, Wade Iii JL, et al. Assessment of infusional 5-fluorouracil schedule and dose intensity: a Southwest Oncology Group and Eastern Cooperative Oncology Group study. Clin Colorectal Cancer. 2005;5(2):119-123.
doi pubmed - Mayer IA, Zhao F, Arteaga CL, Symmans WF, Park BH, Burnette BL, Tevaarwerk AJ, et al. Randomized phase III postoperative trial of platinum-based chemotherapy versus capecitabine in patients with residual triple-negative breast cancer following neoadjuvant chemotherapy: ECOG-ACRIN EA1131. J Clin Oncol. 2021;39(23):2539-2551.
doi pubmed pmc - Meydan N, Kundak I, Yavuzsen T, Oztop I, Barutca S, Yilmaz U, Alakavuklar MN. Cardiotoxicity of de Gramont's regimen: incidence, clinical characteristics and long-term follow-up. Jpn J Clin Oncol. 2005;35(5):265-270.
doi pubmed - Naredi P, Oman M, Blind PJ, Lindner P, Gustavsson B, Hafstrom L. A comparison between hepatic artery ligation and portal 5-Fu infusion versus 5-Fu intra arterial infusion for colorectal liver metastases. Eur J Surg Oncol. 2003;29(5):459-466.
doi pubmed - Ngan SY, Burmeister BH, Fisher R, Rischin D, Schache DJ, Kneebone A, MacKay JR, et al. Early toxicity from preoperative radiotherapy with continuous infusion 5-fluorouracil for resectable adenocarcinoma of the rectum: a Phase II trial for the Trans-Tasman Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2001;50(4):883-887.
doi pubmed - Nobile MT, Sertoli MR, Bruzzone M, Tagarelli G, Rubagotti A, Rosso R. Phase II study with high-dose N5-10-methyltetrahydrofolate and 5-fluorouracil in advanced colorectal cancer. Eur J Cancer Clin Oncol. 1985;21(10):1175-1177.
doi pubmed - Oman M, Lundqvist S, Gustavsson B, Hafstrom LO, Naredi P. Phase I/II trial of intraperitoneal 5-Fluorouracil with and without intravenous vasopressin in non-resectable pancreas cancer. Cancer Chemother Pharmacol. 2005;56(6):603-609.
doi pubmed - Poorter RL, Peters GJ, Bakker PJ, Taat CW, Biermans-van Leeuwe DM, Codacci-Pisanelli G, Noordhuis P, et al. Intermittent continuous infusion of 5-fluorouracil and low dose oral leucovorin in patients with gastrointestinal cancer: relationship between plasma concentrations and clinical parameters. Eur J Cancer. 1995;31A(9):1465-1470.
doi pubmed - Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663-673.
doi pubmed - Regazzoni S, Pesce G, Marini G, Cavalli F, Goldhirsch A. Low-dose continuous intravenous infusion of 5-fluorouracil for metastatic breast cancer. Ann Oncol. 1996;7(8):807-813.
doi pubmed - Rosso R, Mazzei T, Sobrero A, Mini E, Cartei G, Conte P, Labianca R, et al. Phase II trial of 5-fluorouracil and the natural l isomer of folinic acid in the treatment of advanced colorectal carcinoma. Eur J Cancer. 1994;30A(3):338-343.
doi pubmed - Schober C, Papageorgiou E, Harstrick A, Bokemeyer C, Mugge A, Stahl M, Wilke H, et al. Cardiotoxicity of 5-fluorouracil in combination with folinic acid in patients with gastrointestinal cancer. Cancer. 1993;72(7):2242-2247.
doi pubmed - Schuster D, Heim ME, Dombernowski P, Wood C, Queiber W. Prospective multicenter phase-ill trial of doxifluridine (5’dFUR) versus 5-fluorouracil in patients with advanced colorectal carcinoma. Onkologie. 1991;14(4):333-337.
- Smorenburg CH, de Groot SM, van Leeuwen-Stok AE, Hamaker ME, Wymenga AN, de Graaf H, de Jongh FE, et al. A randomized phase III study comparing pegylated liposomal doxorubicin with capecitabine as first-line chemotherapy in elderly patients with metastatic breast cancer: results of the OMEGA study of the Dutch Breast Cancer Research Group BOOG. Ann Oncol. 2014;25(3):599-605.
doi pubmed pmc - Stockler MR, Harvey VJ, Francis PA, Byrne MJ, Ackland SP, Fitzharris B, Van Hazel G, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;29(34):4498-4504.
doi pubmed - Terzoli E, Garufi C, Zappala AR, Vanni B, Pugliese P, Cappellini GA, Aschelter AM, et al. High-dose chronomodulated infusion of 5-fluorouracil (5-FU) and folinic acid (FA) (FF5-16) in advanced colorectal cancer patients. J Cancer Res Clin Oncol. 2004;130(8):445-452.
doi pubmed - Tsavaris N, Bacoyannis C, Milonakis N, Sarafidou M, Zamanis N, Magoulas D, Kosmidis P. Folinic acid plus high-dose 5-fluorouracil with allopurinol protection in the treatment of advanced colorectal carcinoma. Eur J Cancer. 1990;26(10):1054-1056.
doi pubmed - Tsavaris N, Kosmas C, Vadiaka M, Skopelitis E, Kopteridis P, Pamouki S, Efremidis M, et al. 5-fluorouracil cardiotoxicity is a rare, dose and schedule-dependent adverse event: a prospective study. J BUON. 2005;10(2):205-211.
pubmed - Tsuchida E, Sakai K, Matsumoto Y, Sugita T, Sasamoto R, Yamanoi T, Sueyama H, et al. Concurrent chemoradiotherapy using low-dose continuous infusion of 5-fluorouracil for postoperative regional lymph node recurrence of esophageal squamous cell carcinoma. Esophagus. 2005;2(1):25-31.
doi - Urba SG, Orringer MB, Perez-Tamayo C, Bromberg J, Forastiere A. Concurrent preoperative chemotherapy and radiation therapy in localized esophageal adenocarcinoma. Cancer. 1992;69(2):285-291.
doi pubmed - Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, Bugat R, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19(21):4097-4106.
doi pubmed - van Erning FN, Razenberg LG, Lemmens VE, Creemers GJ, Pruijt JF, Maas HA, Janssen-Heijnen ML. Intensity of adjuvant chemotherapy regimens and grade III-V toxicities among elderly stage III colon cancer patients. Eur J Cancer. 2016;61:1-10.
doi pubmed - van Groeningen CJ, Peters GJ, Pinedo HM. Lack of effectiveness of combined 5-fluorouracil and leucovorin in patients with 5-fluorouracil-resistant advanced colorectal cancer. Eur J Cancer Clin Oncol. 1989;25(1):45-49.
doi pubmed - Wang WS, Chen PM, Chiou TJ, Liu JH, Lin JK, Lin TC, Chen WS, et al. Weekly 24-hour infusion of high-dose 5-fluorouracil and leucovorin in patients with advanced colorectal cancer: Taiwan experience. Jpn J Clin Oncol. 1998;28(1):16-19.
doi pubmed - Weh HJ, Wilke HJ, Dierlamm J, Klaassen U, Siegmund R, Illiger HJ, Schalhorn A, et al. Weekly therapy with folinic acid (FA) and high-dose 5-fluorouracil (5-FU) 24-hour infusion in pretreated patients with metastatic colorectal carcinoma. A multicenter study by the Association of Medical Oncology of the German Cancer Society (AIO). Ann Oncol. 1994;5(3):233-237.
doi pubmed - Wenzel C, Locker GJ, Schmidinger M, Mader R, Kramer G, Marberger M, Rauchenwald M, et al. Capecitabine in the treatment of metastatic renal cell carcinoma failing immunotherapy. Am J Kidney Dis. 2002;39(1):48-54.
doi pubmed - Yang TS, Hsu KC, Chiang JM, Tang R, Chen JS, Changchien CR, Wang JY. A simplified regimen of weekly high dose 5-fluorouracil and leucovorin as a 24-hour infusion in patients with advanced colorectal carcinoma. Cancer. 1999;85(9):1925-1930.
doi pubmed - Yang TS, Hsu KC, Wang HM, Lin YC. Phase II study of a weekly 8-hour 5-fluorouracil and leucovorin infusion for patients with advanced colorectal cancer: dose adjusted according to its toxicity. Jpn J Clin Oncol. 2001;31(12):610-615.
doi pubmed - Yang TS, Wang JY, Tang R, Hsu KC, Chen JS. Oral uracil/ftorafur (UFT) plus leucovorin as first-line chemotherapy and salvage therapy with weekly high-dose 5-fluorouracil/leucovorin for the treatment of metastatic colorectal cancer. Jpn J Clin Oncol. 2002;32(9):352-357.
doi pubmed - Ychou M, Duffour J, Kramar A, Debrigode C, Gourgou S, Bressolle F, Pinguet F. Individual 5-FU dose adaptation in metastatic colorectal cancer: results of a phase II study using a bimonthly pharmacokinetically intensified LV5FU2 regimen. Cancer Chemother Pharmacol. 2003;52(4):282-290.
doi pubmed - Yilmaz U, Oztop I, Ciloglu A, Okan T, Tekin U, Yaren A, Somali I, et al. 5-fluorouracil increases the number and complexity of premature complexes in the heart: a prospective study using ambulatory ECG monitoring. Int J Clin Pract. 2007;61(5):795-801.
doi pubmed - Scheithauer W, McKendrick J, Begbie S, Borner M, Burns WI, Burris HA, Cassidy J, et al. Oral capecitabine as an alternative to i.v. 5-fluorouracil-based adjuvant therapy for colon cancer: safety results of a randomized, phase III trial. Ann Oncol. 2003;14(12):1735-1743.
doi pubmed - Narezkina A, Nasim K. Anthracycline Cardiotoxicity. Circ Heart Fail. 2019;12(3):e005910.
doi pubmed - Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin. 2016;66(4):309-325.
doi pubmed - Polk A, Vaage-Nilsen M, Vistisen K, Nielsen DL. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev. 2013;39(8):974-984.
doi pubmed - Mosseri M, Fingert HJ, Varticovski L, Chokshi S, Isner JM. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res. 1993;53(13):3028-3033.
pubmed - Thyss A, Gaspard MH, Marsault R, Milano G, Frelin C, Schneider M. Very high endothelin plasma levels in patients with 5-FU cardiotoxicity. Ann Oncol. 1992;3(1):88.
doi pubmed - Kinlay S, Behrendt D, Wainstein M, Beltrame J, Fang JC, Creager MA, Selwyn AP, et al. Role of endothelin-1 in the active constriction of human atherosclerotic coronary arteries. Circulation. 2001;104(10):1114-1118.
doi pubmed - Spasojevic I, Maksimovic V, Zakrzewska J, Bacic G. Effects of 5-fluorouracil on erythrocytes in relation to its cardiotoxicity: membrane structure and functioning. J Chem Inf Model. 2005;45(6):1680-1685.
doi pubmed - Spasojevic I, Jelic S, Zakrzewska J, Bacic G. Decreased oxygen transfer capacity of erythrocytes as a cause of 5-fluorouracil related ischemia. Molecules. 2008;14(1):53-67.
doi pubmed pmc - Muneoka K, Shirai Y, Yokoyama N, Wakai T, Hatakeyama K. 5-Fluorouracil cardiotoxicity induced by alpha-fluoro-beta-alanine. Int J Clin Oncol. 2005;10(6):441-443.
doi pubmed - Layoun ME, Wickramasinghe CD, Peralta MV, Yang EH. Fluoropyrimidine-induced cardiotoxicity: manifestations, mechanisms, and management. Curr Oncol Rep. 2016;18(6):35.
doi pubmed - Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8(2):191-202.
doi pubmed - Basselin C, Fontanges T, Descotes J, Chevalier P, Bui-Xuan B, Feinard G, Timour Q. 5-Fluorouracil-induced Tako-Tsubo-like syndrome. Pharmacotherapy. 2011;31(2):226.
doi pubmed - Rezkalla S, Kloner RA, Ensley J, al-Sarraf M, Revels S, Olivenstein A, Bhasin S, et al. Continuous ambulatory ECG monitoring during fluorouracil therapy: a prospective study. J Clin Oncol. 1989;7(4):509-514.
doi pubmed - Chakwop Ngassa H, Elmenawi KA, Anil V, Gosal H, Kaur H, Mohammed L. Abnormal dihydropyrimidine dehydrogenase activity as an indicator of potential 5-fluorouracil linked cardiotoxicity in colorectal cancer patients: are toxic events inevitable? Cureus. 2021;13(9):e17712.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.