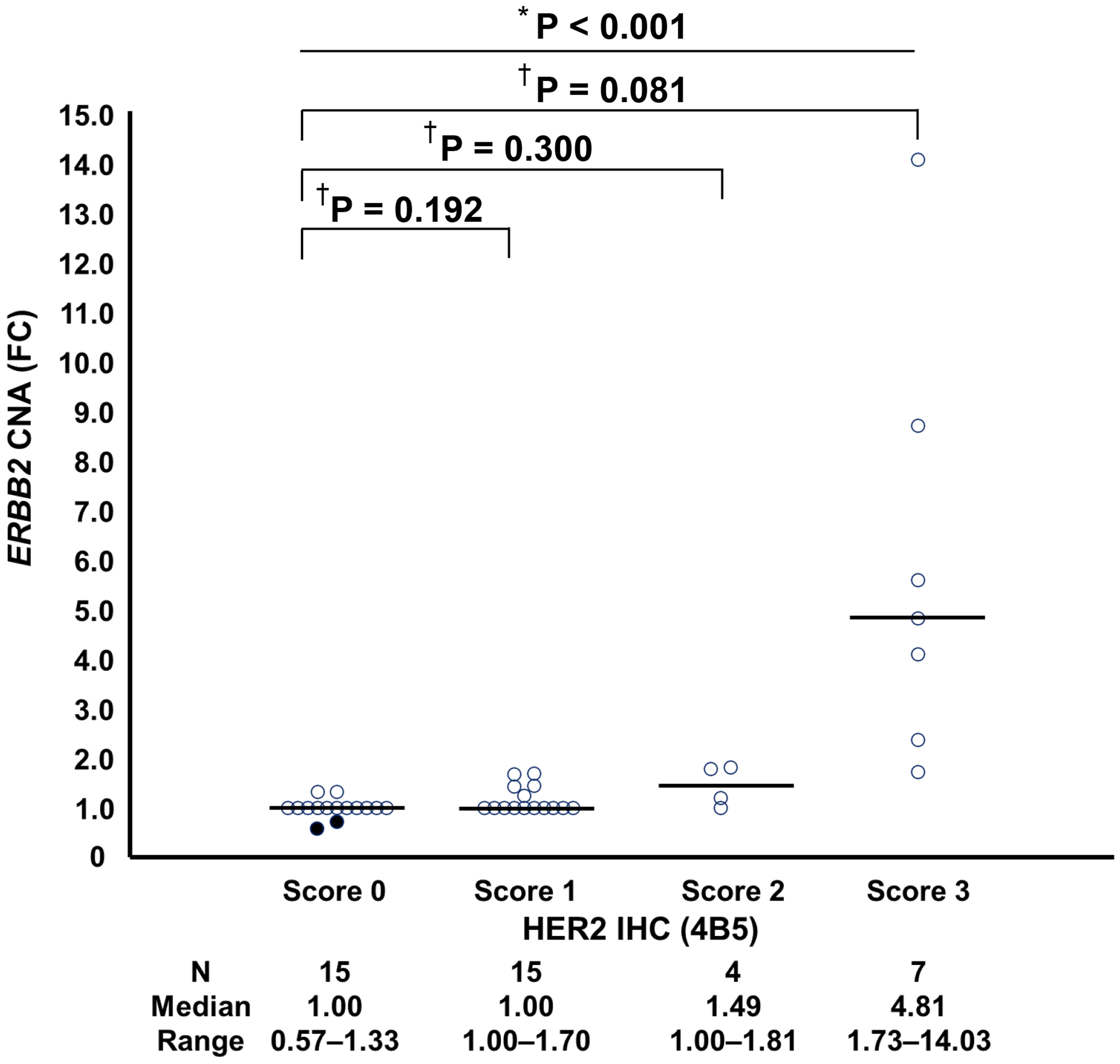
Figure 1. Distribution of ERBB2 CNAs according to HER2 IHC (4B5) expression status. Comparison of ERBB2 CNAs (FC) among patients with HER2 IHC (4B5) scores of 3+, 2+, 1+, and 0. The Kruskal-Wallis test was performed to assess the significance of differences in continuous data among the multiple groups (*). The Games-Howell test was applied to the post hoc tests for multiple comparisons (†). All tests were two-tailed, and P < 0.05 was considered significant. CNA: copy number alteration; ERBB2: erb-b2 receptor tyrosine kinase 2; FC: fold change; HER2: human epidermal growth factor receptor 2; IHC: immunohistochemistry.
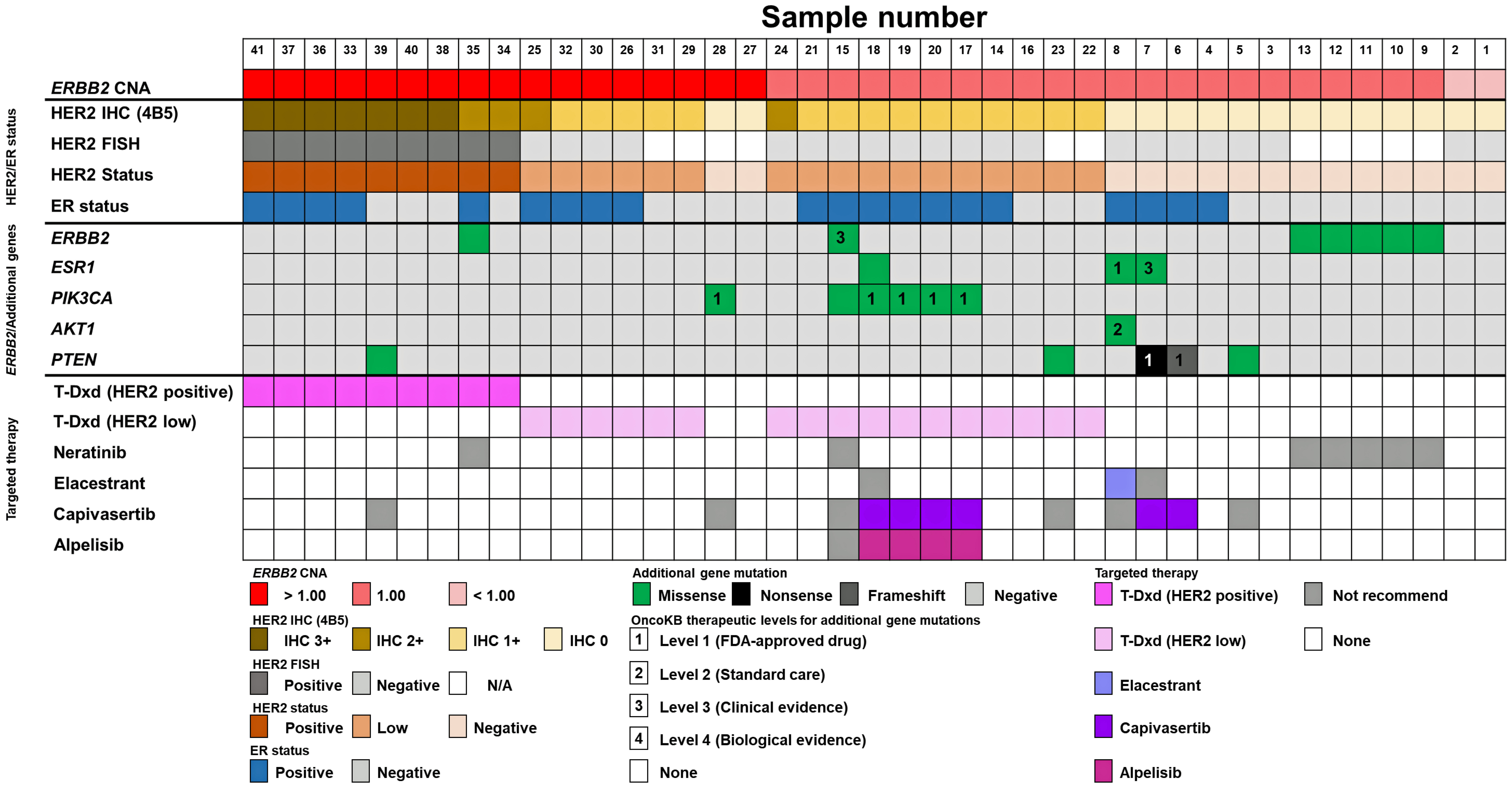
Figure 2. Landscape of additional gene mutations and proposed targeted therapies by targeted NGS, HER2, and ER status. ERBB2 CNA, HER2 status determined by HER2 IHC (4B5) and FISH, ER status, and additional gene mutations in ERBB2, ESR1, PIK3CA, AKT1, and PTEN along with the recommended targeted therapies based on OncoKB therapeutic evidence levels are depicted in color tiles. AKT1: serine/threonine kinase 1; CNA: copy number alteration; ER: estrogen receptor; ERBB2: erb-b2 receptor tyrosine kinase 2; FISH: fluorescence in situ hybridization; HER2: human epidermal growth factor receptor 2; IHC: immunohistochemistry; NGS: next-generation sequencing; PTEN: phosphatase and tensin homolog.
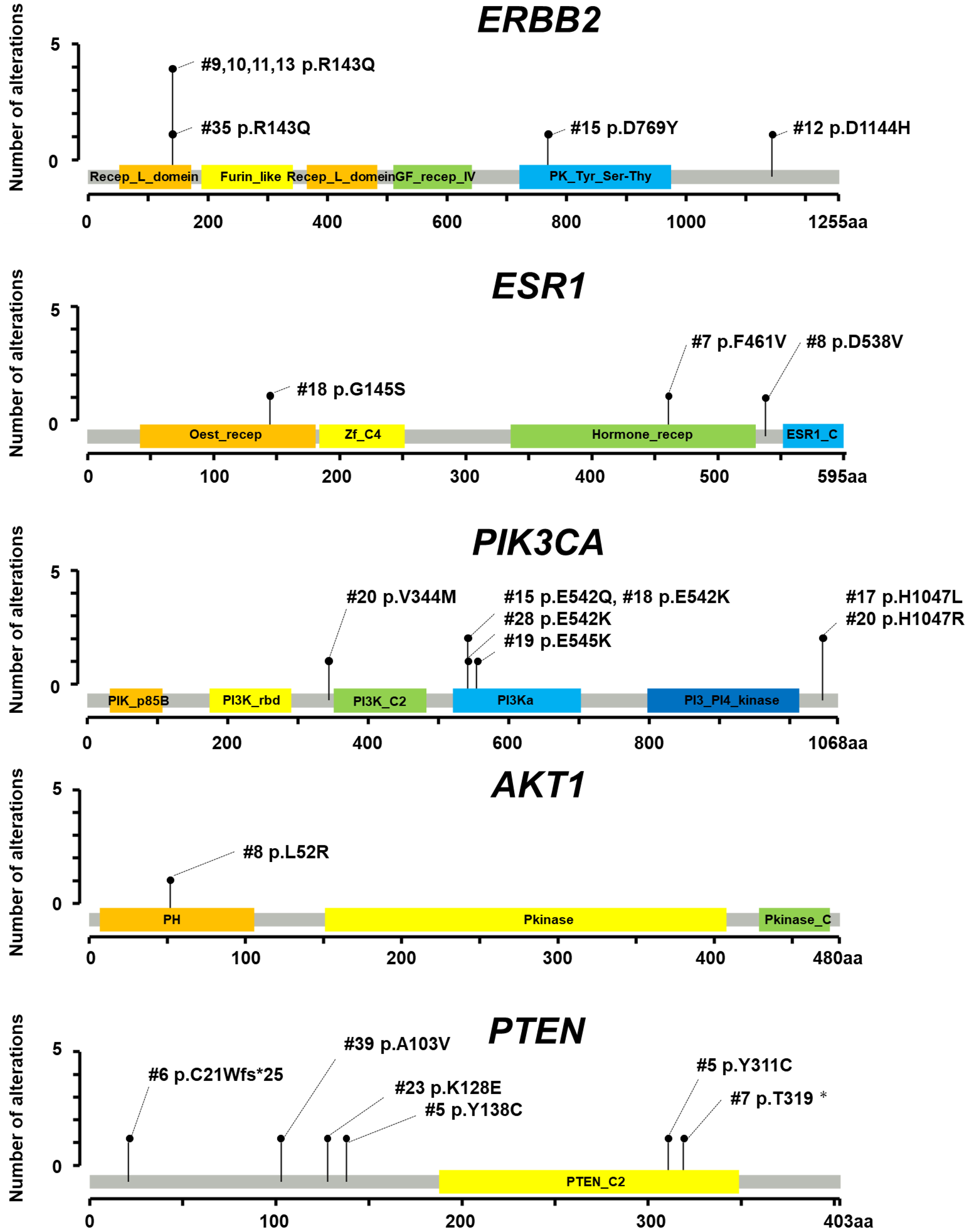
Figure 3. Mutations in ERBB2, ESR1, PIK3CA, AKT1, and PTEN detected in 41 patients. Lollipop plots, which show amino acid changes, are illustrated for ERBB2, ESR1, PIK3CA, AKT1, and PTEN. AKT1: serine/threonine kinase 1; ERBB2: erb-b2 receptor tyrosine kinase 2; ESR1: estrogen receptor 1; HER2: human epidermal growth factor receptor 2; IHC: immunohistochemistry; PIK3CA: phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; PTEN: phosphatase and tensin homolog.


